Before we get into the event of the week – the ASCO abstract drop on Wednesday evening – lets take a look at what’s been going on with the biotechnology sector as a whole.
The iShares Biotechnology Index ETF (IBB) has been making higher lows for just over a month now as it hugs its 200-day simple moving average. But the ETF is approaching a major resistance line that marks the downtrend for the security since this correction began in March. With the ETF winding tighter, a break in one direction or the other is imminent.
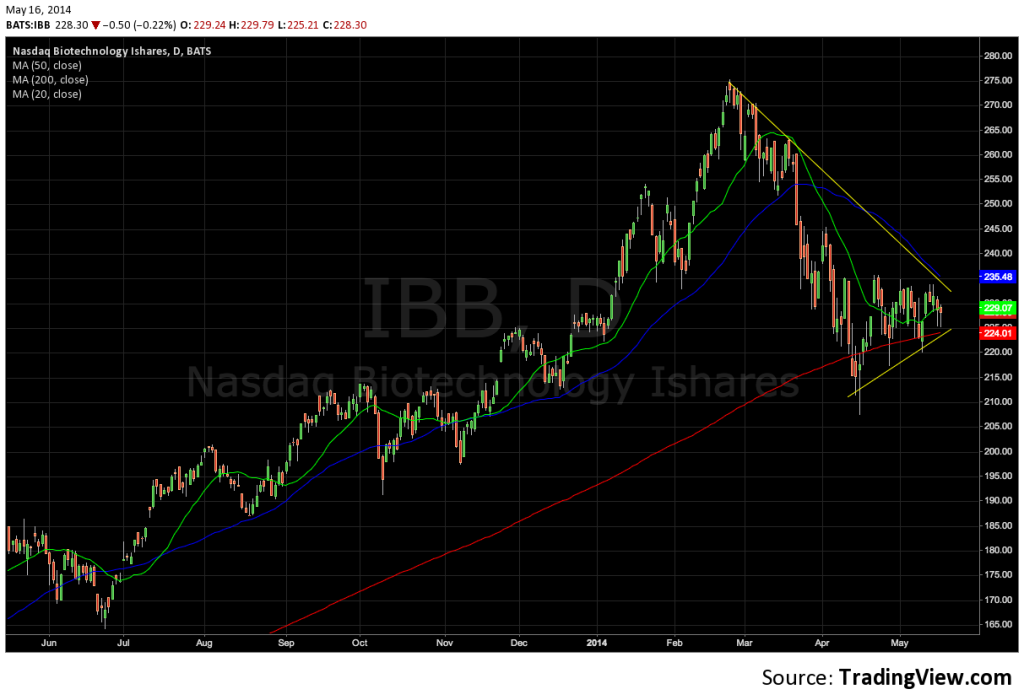 A move above the yellow downtrend would be a positive development, signaling that the trend, at least in the short-term, has been broken – the transverse is true if the resistance holds up.
A move above the yellow downtrend would be a positive development, signaling that the trend, at least in the short-term, has been broken – the transverse is true if the resistance holds up.
Risk has been coming out of the sector all quarter, but generalists are still eyeing the broader market – the S&P 500, the DOW, the NASDAQ – with trepidation, even as the two indices made new all-time highs this week. Josh Brown of The Reformed Broker posted an interesting discussion of the small-cap, large-cap divergence of breadth and price last week – it’s worth a read.
Following up on our pre-abstract ASCO preview, PropThink revisited some of the companies with noteworthy new data, or a lack of, that moved the market this week. Shares of Incyte (INCY) dropped following a somewhat disappointing “big reveal” related to its lead and already approved cancer drug, Jakafi (ruxolitinib). Analysts and investors have been waiting for more information on a subset of pancreatic cancer patients that performed well on Incyte’s drug. Underwhelming high expectations, the subset was made up of patients with inflammation, measured by levels of C-reactive protein. Meanwhile, Clovis Oncology (CLVS) jumped on evidence that its lung cancer drug, CO-1686, may be safer than a competing candidate from AstraZeneca (AZN). We also revisited one micro-cap company that we think is worth knowing into the end of the month and the annual meeting. Read the full story, here.
We’ve watched shares of Orexigen Therapeutics (OREX) fall since late March, and the stock is now 30% off of recent highs on the broader sector weakness. The equity has fallen significantly in front of a key inflection point, the June 10 approval decision for the weight loss drug NB32. PropThink Premium subscribers can get our take on OREX, NB32 (Contrave), and the future for the prescription weight-loss market in our latest research: Obesity Deja Vu: Orexigen’s Approval Decision is Next Month.
Get your first month of PropThink Premium for 50% off.
Sarepta Therapeutics (SRPT) will file a New Drug Application for eteplirsen, its lead drug, in Duchenne Muscular Dystrophy by the end of 2014. All-in, the company is preparing to start six new studies in the next ten months for a platform approach to treating DMD that could be worth billions. There are a lot of moving parts to Sarepta’s 2014, and on Tuesday following a company webcast to update the DMD community, Mr. King broke down the company’s next few moves.
One or more of PropThink’s contributors are long INCY, OREX, or SRPT.





