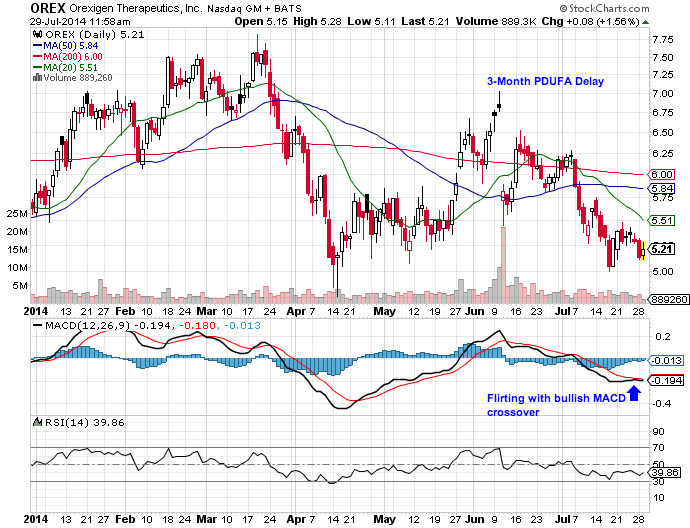
Orexigen Therapeutics (OREX) has again dipped below $5.50, which we find attractive 6 weeks ahead of the September 11 PDUFA decision for NB32 (Contrave, naltrexone/bupropion), and an opinion in Europe not long after. In addition, some lesser-known events in the next month could have a meaningful read-through on NB32’s commercial viability.
We last suggested owning OREX in May, shortly before the scheduled June 11th PDUFA decision for NB32, as the stock traded around $5.40. One day before the PDUFA, on June 11 (rescheduled), we took the trade off when OREX topped $7.00. Subsequently, the FDA again delayed the PDUFA decision, this time to September 11, citing agreements on post-marketing obligations related to the ongoing cardiovascular outcomes trial, dubbed the LIGHT study.
- Buy OREX between $5.00 and $5.50.
- Look for stock to return to pre-PDUFA highs around $6.50-7 before Sept 11. Selling this range makes sense, and consider buying weakness on FDA approval (Sept. 11) as European decision comes into view in the September/October timeframe.
- Read more about Orexigen here.
One Theory on the Delay
The 3-month PDUFA extension was unexpected given the positive interim outcome from the LIGHT study and a Special Protocol Assessment in place.
The interim look late last year (November) was designed to rule out a doubling of cardiovascular risk (stroke, heart attack) in patients on Contrave compared to placebo, and the quantitative results of the interim analysis serve as a component of the NDA package and European MAA. The trial calls for full data to remain blinded until the trial is complete in 2017.
The delay may, however, be related to an ongoing discussion between Orexigen and FDA on the possibility of unblinding the interim results, beneficial to Orexigen in that it could use the results, if positive, in marketing material. On a conference call held when Orexigen announced the delay, the company cited two examples of similar situations:
…the need for dialogue with the agency on these matters has occurred in other recent reviews of diabetes drugs, where sponsors used interim data from ongoing blinded trials to pursue approval. In one of these cases the ongoing trial continued and fulfilled the post marketing requirement. This has been the plan for Contrave and the Light Study. [SVP of Development, Klassen, is referring to Nesina (alogliptin)]
In another case the trial was unblinded after the interim analysis and a new trial formed the basis of the post marketing requirement. In both cases data disclosure was an important topic discussed in the review, and ultimately agreement was reached regarding post marketing obligations. [Klassen is most likely referring to Invokana (canagiflozin)]
Invokana (canagliflozin) was approved in March of 2013 as the first sodium-glucose co-transporter 2 (SGLT2) inhibitor indicated for adults with type 2 diabetes mellitus (T2DM). Jansen (JNJ), before the approval, was running a long-term cardiovascular outcomes trial called CANVAS, with an interim analysis designed to exclude a 1.8 risk margin; the trial was to remain blinded following the interim. The interim results, however, were somewhat confounding. The drug produced a higher rate of CV events during the first 30 days of treatment, but no imbalance following. In order to gain insight into this effect, the data were discussed openly at a January FDA advisory committee meeting. The drug was approved two months later, though regulators required further post-approval testing of CV signal. Jansen and FDA settled on continuation of CANVAS after approval, with the introduction of CANVAS-R, which includes CV events as a non-primary endpoint.
Orexigen on its post-delay conference call essentially broached the subject of unblinding the ongoing LIGHT study, which would probably require alteration to the post-marketing plan.
Supporting the thesis that the delay is related to public release of the LIGHT interim results – an upcoming Public Hearing in front of The Commissioner at the FDA has been scheduled for August: Confidentiality of Interim Results in Cardiovascular (CV) Outcomes Safety Trials. The purpose of the meeting is “to initiate constructive discussion among regulators, researchers, health care providers, representatives from the pharmaceutical industry and health care organizations, and the general public, about appropriate handling of interim analysis results of these ongoing CVOTs,” and the register specifically notes CVOT trials for obesity treatments, as well as diabetes products.
The event is scheduled for August 11, and an agenda is due in the 2 weeks before.
Exactly what to expect from the public hear is not clear; however, we’re looking for a strong presence from representatives of Orexigen. If Orexigen presents at the August meeting and petitions the FDA to allow unblinding of interim results from CV outcomes studies, it stands to reason that the company is sitting on quality data; in the best-case scenario, NB32 produced a lower rate of CV events than placebo in the LIGHT study. Clearly, Orexigen would want to use this information in marketing NB32 to physicians, which it cannot do if the interim results remain blinded. Remember, all we know thus far is that NB32 did not double the risk of major cardiovascular events compared to placebo. This information would also support the drug’s approval in the U.S. and E.U. and explain Orexigen’s effusive confidence in recent press releases and public appearances.
The August event will be webcast live, here, and an agenda will be posted here. (guidance is for 2 weeks in advance of the meeting).
Also upcoming is an approval decision in Europe. Orexigen should have received the Committee for Medicinal Products for Human Use’s (CHMP) 180-day List of Outstanding Issues (LoOI) by the end of July. Assuming no Oral Explanation is required and a 30-day response to CHMP, Orexigen should receive an opinion in September or October. We lean towards the latter.
Orexigen typically reports second quarter earnings early in the 2nd week of August, which we’ll be watching for an update on the LoOI or even the public hearing.
The Alternative
The above is one hypothesis. A delay this late in the review cycle is disconcerting, particularly with an SPA in place for the LIGHT study. This is part of the reason we suggest taking the trade off in the $6.00-$7 range ahead of the PDUFA decision; NB32’s approval is likely to be a sell-the-news event. Again, a dip following approval may offer an attractive place to get long yet again in front of the EU decision.
Upcoming Milestones:
July 28 – August 11. Agenda for upcoming public hearing. (Check here)
2nd Week of August. Q2 Earnings, possibly an update on 180 day List of Outstanding Issues from EMA/CHMP
August 11. Public Hearing on the Confidentiality of Interim Results in Cardiovascular (CV) Outcomes Safety Trials
September 11. Scheduled PDUFA Decision
September or October. CHMP opinion.
One or more of PropThink’s contributors are long OREX.