Zogenix (ZGNX) is divesting of its lead FDA-approved product, the painkiller Zohydro, for $100 million ($30 million in cash up front) and almost $300 million in potential milestone payments. As much as the cash and milestone potential are important to Zogenix, which is re-positioning itself as a pipeline story, more important is that Zogenix is cutting loose the albatross around its neck for the last year and a half.
The specialty pharmaceutical company Pernix Therapeutics (PTX) will pay $30 million in cash, $20 million in company stock, and a $50 million short-term promissory note to buy Zohydro outright. Pernix will also take Zogenix’s ~100-person salesforce, and will potentially owe to Zogenix $284 million in sales and development milestones related to the Zohydro franchise.
Zogenix reported Zohydro ER gross factory sales to wholesalers of $9.2 million during the fourth quarter of 2014, $27.3 million for the entire year.
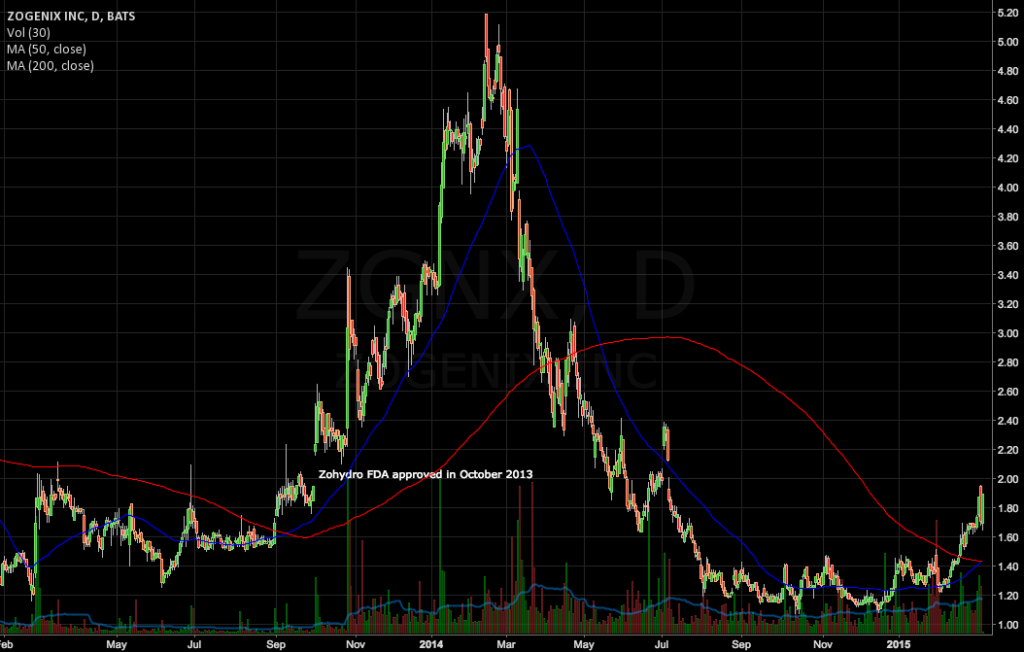
Zohydro was approved in October of 2013 as the first extended-release hydrocodone product without acetaminophen for the management of severe pain. But in the 1.5 years since, outcry from practitioners, legislators, and even patient-advocacy groups over the potential for Zohydro ER to be abused (which is in opioid but contained no abuse-deterrent qualities) has weighed on the stock. A newer version, approved in January of 2015, reduces the risk of abuse to some degree, but ZGNX has continued to suffer.
Here’s the ZGNX chart going back two years. Zogenix’s stock is down from the day Zohydro was approved.
By divesting of Zohydro, Zogenix brings in some non-dilutive cash and hopes to focus investors back on the pipeline, two products in development for the rare form of epilepsy known as Dravet Syndrome, and schizophrenia. ZX008 will enter Phase 3 development in Dravet Syndrome this year, and Relday (long-acting, injectable risperidone) will enter Phase 3 studies next year, according to Zogenix.
Zogenix expects its existing cash ($42.2 million at year-end), $20 million in term loans & $4 million in revolving line commitments, plus proceeds from the Zohydro ER sale to last through the fourth quarter of 2016.
It remains to be seen whether investors will get on board with the company’s new tack.