Vericel Corp (VCEL) cratered on Monday with details from a phase 2b study of its ixmyelocel-T cell therapy for heart failure, presented at the American College of Cardiology’s (ACC) 65th Annual Scientific Session.
Three weeks back, Vericel announced via press release that the phase 2b trial has been complete, with positive data. The stock climbed 50% initially, and doubled again in the following two weeks. At the time, Vericel offered no data from the study, except to note that the trial met its primary endpoint: treatment with ixmyelocel-T resulted in a reduction in the total number of deaths, cardiovascular hospitalizations or unplanned outpatient and emergency department visits in the 12 months following treatment, compared to placebo.
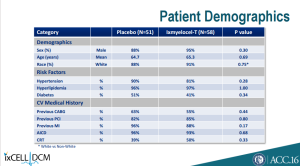
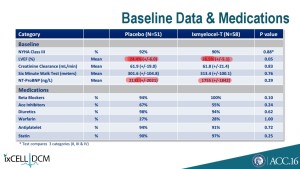
With details at ACC this Monday, investors aren’t so optimistic about the product’s commercial potential, sending the stock lower by 30%. First, baseline imbalances favoring placebo patients almost across the board could explain much, if not the entirety of, the out-performance of ixmyelocel-T-treated patients:
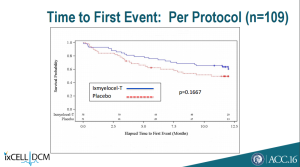
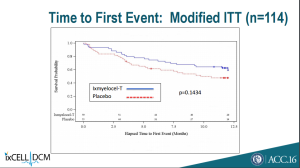
Additionally, ixmyelocel-T failed to beat placebo on a number of secondary endpoints.
All of the sudden, ixmyelocel-T looks quite a bit less marketable. Find the full ACC presentation here.