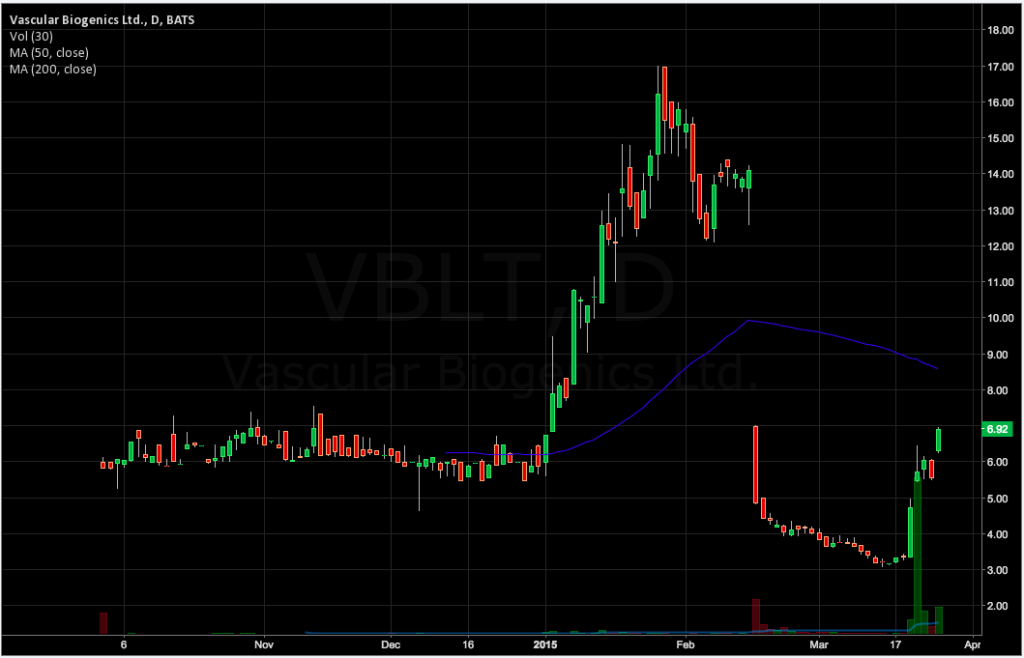
VBL Therapeutics (VBLT) claimed success in a trial of lead drug candidate VB-111 in brain cancer on Wednesday, sending the stock higher by 40% in early trading. At the open, VBL was up 15% to $6.50. The FDA removed a clinical hold on VB-111 in February, and the company plans to begin a phase 3 trial in glioblastoma around mid-year.
We wrote about VBL’s odd history just a month ago.
The released topline VB-111 results suggest an improvement in overall survival in patients with recurrent glioblastoma (rGBM) who were treated with a combination of VB-111 and bevacizumab (Avastin), compared to patients treated with bevacizumab alone.
Of 46 rGBM patients in the trial, 22 were treated with VB-111 monotherapy, which was discontinued upon disease progression, before beginning treatment with bevacizumab alone. Meanwhile, 23 patients received a combination of VB-111/bevacizumab after progression on BV-111 monotherapy; 1 patient remained stable on VB-111 monotherapy. The results suggest improvement in overall survival for those receiving the combination therapy, with a median overall survival of 414 days compared to 235 days in the VB-111-to-bevacizumab-monotherapy arm (p=0.05).
The results focus on only 46 patients, a significantly smaller group than the planned enrollment of 90 patients (as reported on www.clinicaltrials.gov). Additionally, results on the primary outcome – progression free survival – were not released. The trial remains open to enrollment despite having an estimated primary completion date of January 2015.