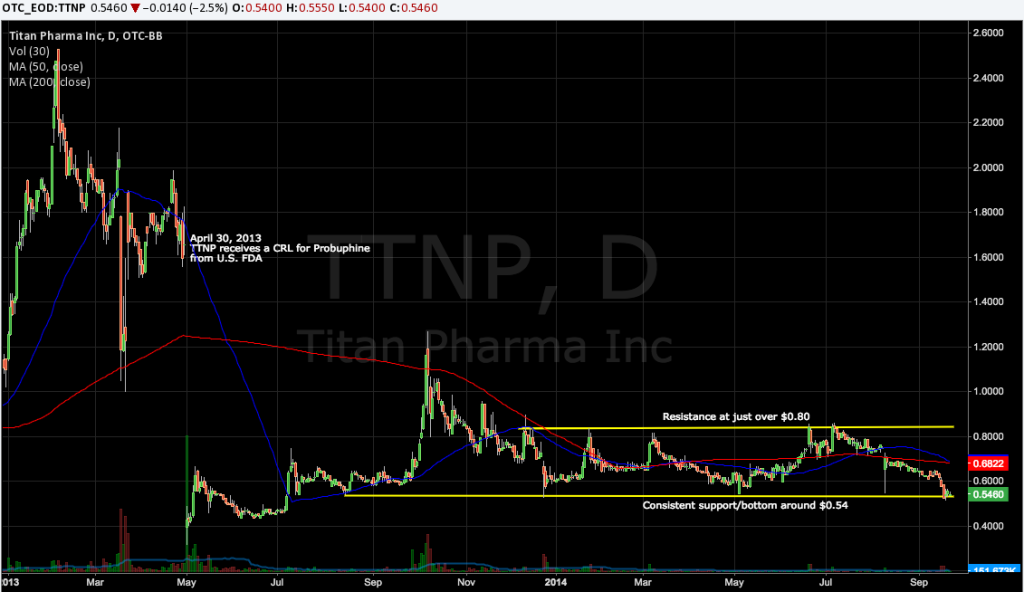
Titan Pharmaceuticals (TTNP) announced on Tuesday that enrollment in a final phase 3 trial of Probuphine has reached the halfway mark, and that the company continues to expect results by mid-2015. Probuphine is Titan’s subdermal buprenorphine implant for the maintenance treatment of opioid dependence. Though TTNP’s next big event is still 9 months away, it’s worth noting that TTNP is trading at the bottom of a year-long price-range, and Tuesday’s news may be all it takes to put the stock in motion higher.
The phase 3 study, being run by Titan’s partner Braeburn Pharmaceuticals, is designed to support resubmission of the New Drug Application for Probuphine to the U.S. FDA, which the company expects later in 2015.
Titan received a CRL for Probuphine in April of 2013, with the FDA requesting additional data on the effect of higher doses of Probuphine that would more closely approximate the blood plasma levels associated with sublingual doses of buprenorphine; and human factor testing, among other requests. The current phase 3 tests Probuphine vs. sublingual buprenorphine in clinically stable patients who are receiving maintenance treatment with sublingual buprenorphine at a daily dose of 8mg or less. Patients are randomized to receive either four Probuphine implants, or to continue the daily sublingual buprenorphine therapy. 183 patients will be treated for six months, and the primary analysis is a non-inferiority comparison of responders in the two arms.
Titan needs capital, which has depressed the stock for months. Existing cash and equivalents of $8.9 million at June 30 should last into mid-2015, according to management. We suspect TTNP orchestrates some sort of financing with partner Braeburn (given the larger company’s substantial stake in TTNP), but until a deal gets done the financing overhang will remain.
You can read some of PropThink contributor Jason Napodano’s older TTNP coverage by clicking here.