In the wake of a sentiment shift for Arrowhead Research (ARWR) and its Hepatitis B therapeutic, ARC-520, Tekmira Pharmaceuticals (TKMR) presented an encouraging first look at its own early-stage treatment, TKM-HBV, on Wednesday at the 10th Annual Meeting of the Oligonucleotide Therapeutics Society.
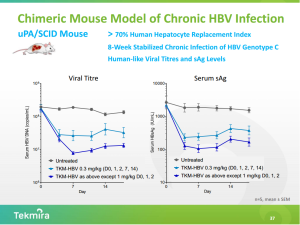
Tekmira reported results in a variety of preclinical models, including potent reduction of Hepatitis B surface antigen (HBsAg) and cccDNA reduction in the uPA/SCID chimeric mouse model. This mouse model is unique compared to other animal models in which experimental HBV therapeutics have been tested to date, including those used by Arrowhead, because it has the ability to support cccDNA, an important molecule that acts as factory and reservoir for the HBV virus. Alnylam’s (ALNY) ALN-HBV and Arrowhead’s ARC-520 both lack preclinical models that demonstrate an ability to reduce cccDNA. It’s important to note the validity of cccDNA generated from mouse models and its translation into NHPs and humans is still controversial, but cccDNA is important because a cure for HBV requires full clearance of cccDNA and HBV DNA from the body.
Tekmira’s TKM-HBV preclinical data package builds on 2nd generation hepatitis B siRNA-LNP SNALP (ALN-HBVLNP) studies in non-human primates generated by Sirna Therapeutics, which was acquired wholly by Alnylam earlier this year. Alnylam’s HBV therapeutic, ALN-HBVLNP, demonstrated a mean 0.7log10 (80%) knockdown of HBsAg with a single 0.25 [mg/kg] dose in non-human primates (N=4). For reference, Arrowhead’s ARC-520 (Dynamic Polyconjugates) produced a 0.7log10 knockdown using 2 [mg/kg] and 3 [mg/kg] in a non-human primate (N=1) and is being dosed at 2-4 [mg/kg] in ongoing clinical trials.
TKM-HBV uses a more potent 3rd generation SNALP and UsiRNA triggers. TKM-HBV achieved a rapid 1log10 HBsAg knockdown at 1 [mg/kg] and demonstrated the ability to reduce cccDNA in the uPA/SCID model. It’s important to understand that extrapolating these mouse model results to non-human primates or humans is difficult and dangerous – both models underestimate potency and duration of effect.
Notably, to achieve 0.7log10 HBsAg knockdown in NHPs, Arrowhead used doses that are higher than those used in current clinical trials, while TKM-HBV appears able to achieve the same knockdown at a single dose within their therapeutic window – the range of SNALP doses already utilized in humans. By referring to previous ALN-HBVLNP data, one might assume that TKM-HBV is more potent than ALN-HBVLNP and ARC-520 since it uses more potent 3rd generation SNALP and more potent UsiRNAs.
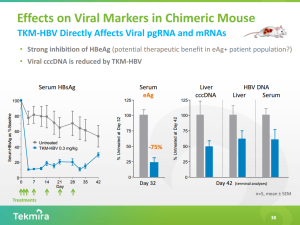
My suspicion is that TKM-HBV has the ability to demonstrate over 1log10 HBsAg reduction following a single dose in non-human primates, or humans. The standard of care for chronic HBV infection, Entecavir + Interferon-alpha, does not rapidly reduce HBsAg and does not reduce cccDNA in the uPA/SCID chimeric mouse model (Link). Unlike Entecavir or Interferon-alpha, TKM-HBV has a mechanism of action that can potentially lead to a cure for chronic HBV by reducing HBsAg and cccDNA. Again, the HBsAG thesis has yet to prove out in humans.
One or more of PropThink’s contributors are long TKMR.