Sunesis Pharmaceuticals (SNSS) announced Monday much-anticipated results from the pivotal Phase 3 VALOR trial, a randomized, double-blind, placebo-controlled trial of vosaroxin (trademark Qinprezo) and cytarabine in patients with first relapsed or refractory acute myeloid leukemia (AML).
The trial, which enrolled 711 patients at more than 100 international sites, did not meet its primary endpoint of demonstrating a statistically significant improvement in overall survival, with a median overall survival of 7.5 months for vosaroxin + cytarabine compared to 6.1 months for placebo and cytarabine (p=0.06). The secondary endpoint, a clinically significant benefit in complete remission (CR) rate (30.1% vs 16.3%, p=0.0000148), was met. Analysis of overall survival censoring for stem cell transplantation of patients aged 60 years and older (n=451), where the rate of stem cell transplant was 20.2%, the vosaroxin combination demonstrated a median overall survival of 7.1 months, versus 5.0 months for placebo and cytarabine (p=0.006), and a CR rate of 31.9% versus 13.8% (p=0.0000048).
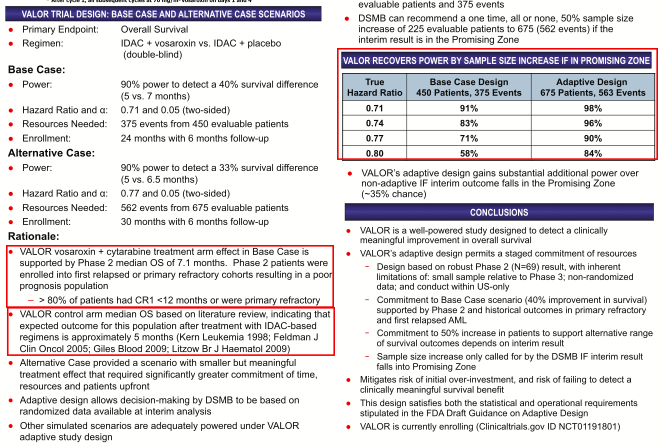
Cytarabine clearly outperformed the company’s early assumptions. The VALOR trial was 84% powered to detect a 25% benefit, designed assuming a median OS for r/r AML patients receiving cytarabine of 5 months, and a mOS of ~7 months for cytarabine in combination with vosaroxin. The SNSS long thesis hinged largely on a 2012 sample size increase for VALOR and improved power with which Sunesis expected to detect (with statistical significance) this OS benefit. Interestingly, it was Monday’s very scenario that SNSS and its investors hoped the phase 3 VALOR trial could avoid: a one-month-plus OS improvement that did NOT produce statistical significance.
Source: Sunesis Pharmaceuticals
Sunesis plans to begin a marketing authorization application with the European Medicines Agency (EMA) and will meet with the FDA to determine the appropriate regulatory path forward. Earlier this year, Joseph I. DePinto was appointed as chief commercial officer to specifically help coordinate a U.S. launch of vosaroxin following the data readout, and the company secured the trademark name Qinprezo for vosaroxin in both the U.S. and the EU.