Amicus Therapeutics (FOLD) reported on Wednesday positive results from Study -012, comparing migalastat monotherapy to standard of care therapy in patients with Fabry Disease. The stock is up 15% in the session, and PropThink Premium subscribers who followed our strategy two weeks ago are up 50% on the position (read it here). FOLD’s reaction on Wednesday is muted because the stock was already up nearly 175% since the beginning of June, not unexpected and part of the reason for our sold Calls.
On August 7, we suggested being involved in FOLD ahead of the -012 data, outlining an options strategy to improve investors’ risk/reward going into the binary event, on which we were bullish. We suggested being long FOLD from $4.10, selling Oct $5 Calls at $1.00, and buying Sept. $2.5 Puts at $0.20, for a cost basis of $3.30. Upside on the trade is capped at 52% ($3.30 to $5.00 via the short Calls) and downside limited to 25% ($3.30 to $2.50 via the purchased Puts).
Migalastat was compared to enzyme replacement therapies (ERTs) Fabrazyme and Replagal in Study 012. The study met both co-primary endpoints, comparing mean annualized changes in estimated glomerular filtration rate (eGFR) and measured GFR (mGFR) assessed by descriptive comparisons over 18 months: A ≥50% overlap in the confidence intervals between the migalastat and ERT treatment groups; AND mean annualized change for patients receiving migalastat was within 2.2 mL/min/1.73 m2/yr of patients receiving ERT.
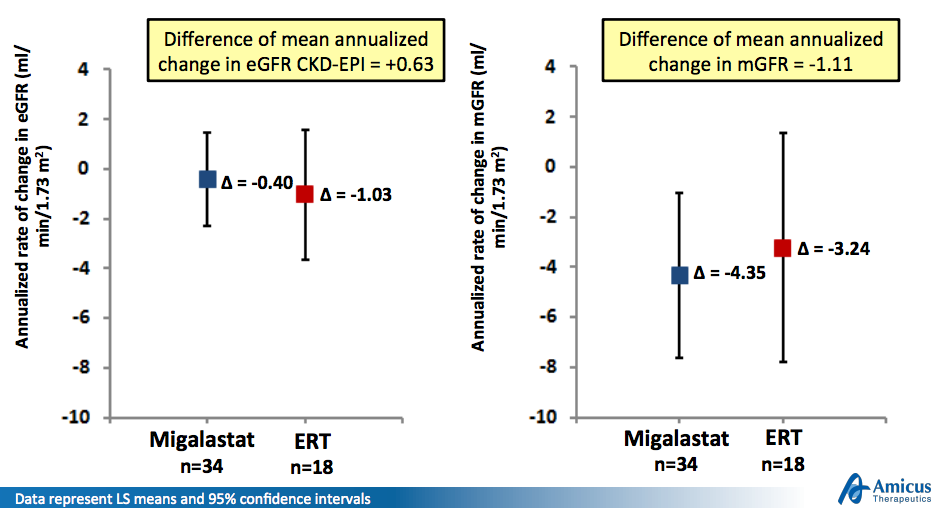
Amicus expects positive results from Study 012 to be sufficient for European approval; FDA approval is still an unknown given a previous phase 3 failure, though the company will meet with the agency in the fourth quarter to discuss a path forward. Subscribe to PropThink Premium to follow our next move with this ongoing trade.

Already a Premium user? Sign In




