Fennec Pharmaceuticals (FENC:NASDAQ) (FRX.TO:TSX) released the results of its long anticipated Phase 3 study. Interim data showed Fennec's drug, sodium thiosulfate ("STS") significantly reduces chemotherapy induced hearing loss without any evidence of tumor protection. The Company also began trading on the NASDAQ under symbol 'FENC', uplisting from the over-the-counter market as we had expected.
Fennec's drug candidate addresses a market worth at least $100m each year and may be an attractive acquisition target given its acute need, regulatory barriers to entry for any competitors, and widespread awareness among practitioners.
Pediatric Cancer Space Has Achieved High Survival Rates, But Severe Long Term Adverse Effects Linger
Fennec is testing Sodium Thiosulfate (STS) in children as a preventive agent against hearing loss caused by cisplatin, a platinum-based chemotherapy. Pediatric tumors generally have a good prognosis with overall survival greater than 80%[1]. However, roughly 60%[2] of children undergoing chemo will experience some degree of permanent hearing loss. This is terribly disabling and becomes a lifelong hardship for young children who are at critical stages of developing language skills and later extends to academic performance and social development.
Figure 1: Although data for patients up to 20 years old has been collected, children under age of 5 face highest probability of chemotherapy induced hearing loss [3]
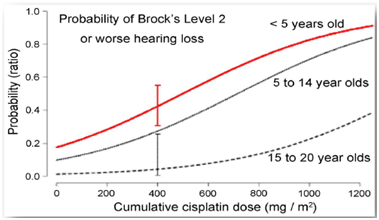
With survival rates over 80%, the focus is on minimizing the long term adverse effects of children undergoing chemo. Currently, the standard of care is the lifelong use of hearing aids or, for children with the most severe hearing loss, cochlear implants. Lifetime costs for these treatments range between $50,000 - $70,000 [4]. While implants provide some benefit, there is a need for a more convenient and permanent solution.
Trials Have Shown Sodium Thiosulfate (STS) Can Reduce Hearing Loss Associated with Platinum-based Chemotherapy
Sodium thiosulfate (STS) is a thiol-containing antioxidant that is excreted bythe kidneys after intravenous administration.STS is administered 6 hours after a cisplatin infusion, allowing for the platinum based chemotherapy to complete its anti-tumor activity. Once administered intravenously, STS binds to cisplatin, forming a non-cytotoxic compound that minimizes damage to healthy cells and is then excreted by the body.
Early stage studies, conducted by investigators at Oregon Health & Science University (OHSU), have shown that STS can reduce the hearing loss associated with platinum-based chemotherapy. Fennec has collaborated with the Children’s Oncology Group (COG) and the International Childhood Liver Tumour Strategy Group (SIOPEL) to conduct two Phase III studies, respectively.
First Phase III Study (COG ACCL0431) [5]: Showed STS worked but may intervene with the anti-tumor activity of the platinum-based chemotherapy
This open label, randomized proof of concept study enrolled 131 children (under 18 years of age) that were newly diagnosed with various childhood cancers. Patients were randomized to receive cisplatin according to their disease-specific regimen + STS (active arm) and cisplatin according to disease-specific regimen (control arm).
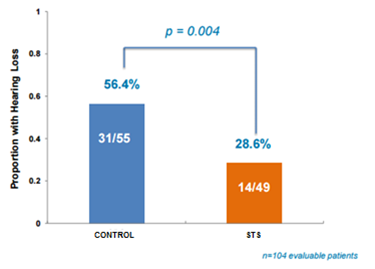
Out of the 104 evaluable patients, hearing loss was identified in 14/49 (28.6%) participants in the STS group compared with 31/55 (56.4%) in control group. The study showed a statistically significant improvement (p=0.004) in proportion of patients with hearing loss, meeting the primary endpoint.
Figure 2: STS helps prevent hearing loss in children undergoing cisplatin chemotherapy
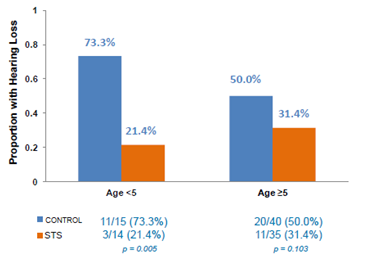
However, it must be noted that when looking at STS efficacy by age, the effect of STS was more pronounced in children under 5 years old, albeit this is a small sample size of just 29 patients. In this randomized arm, hearing loss was identified in 3/14 (21.4%) in STS group compared with 11/15 (73.3%) control group.The significance here is that children under the age of 5 are also the most susceptible to cisplatin-induced hearing loss (shown in Figure 1, above).
Figure 3: STS proved to be most effective in children aged <5 against cisplatin-induced hearing loss
STS Safety Data Skewed by Indirect Control Group Comparison
The secondary endpoints of event free survival (EFS) and overall survival (OS) were measured to track the impact of STS on the platinum-based chemotherapy. EFS for the active group was 54% vs. 65% for the control group (p=0.36), while OS for the active group was 70% vs. 87% for the control group (p=0.07), indicating that STS may intervene with the anti-tumor activity of the cisplatin.
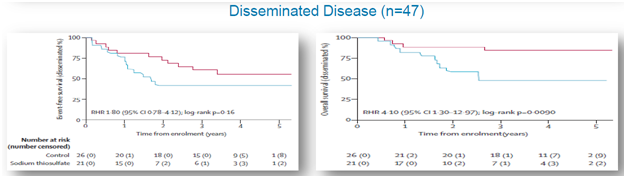
A post hoc analysis was performed that observedEFS and OS for patient subsets of subjects with disseminated (metastatic) and localized cancer. For localized cancer, EFS for the active group was 60% compared to 66%for the control group (p=0.73), while OS for the active group was 83% compared to 89% for the controlgroup (p=0.88), shown below. For metastatic cancer, EFS for the active group was 42% compared to 61% forthe control group (p=0.16), while OS for the active group was 45% and 84% for the control group (p=0.009), shown below.
Figure 4: Data shows that STS is intervening with anti-tumor activity of cisplatin in disseminated cancers
Below is a breakdown of cancer types by arm. The “Others**” tumor type included tumor types that were not represented in the control arm, so not an exact apples to apples comparison. The STS arm had 3 deaths (2 with ATRTs and 1 with Carcinoma) whereas control had 0, skewing the OS downwards compared to control. Although STS arm had a lower OS than control, the different cancer comparison is not a good indication of STS' actual safety.
Figure 5: A deeper dive into the data shows that OS was skewed by STS deaths that were not directly comparable to control group
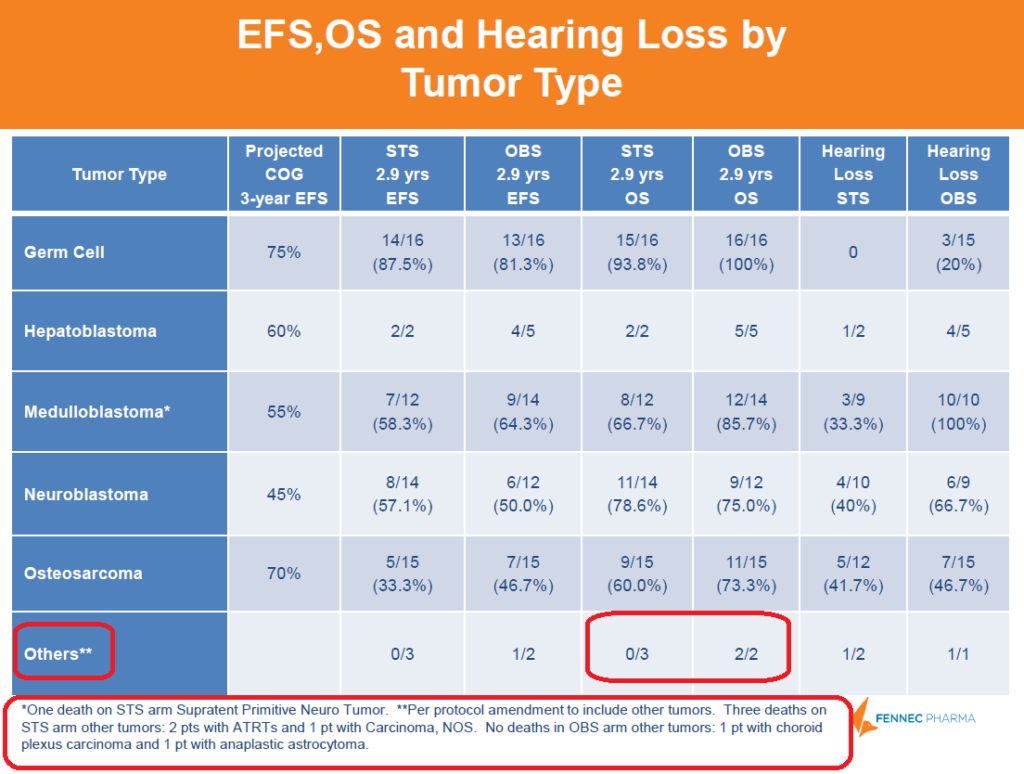
Could STS Safety Concerns Be A Blessing in Disguise?
The questionable safety data in disseminated cancers may prove to be a blessing in disguise for Fennec because it may mean the company applies for the FDA’s Risk Evaluation and Mitigation Strategies (REMS) program. This is a risk management program that manages drugs with potentially serious safety risks and thus requires developers to keep a registry of all patients taking the drug, certify doctors who prescribe the drug and restrict distribution channels.
Companies that fall under this program have historically used the drug to build moats and effectively create monopolies long after Orphan Drug Designation and European Market Exclusivity are expired. Under the REMS program, Fennec can theoretically eliminate generic competition by refusing to sell samples for bioequivalent testing since the generic maker is not a permissible distributor.
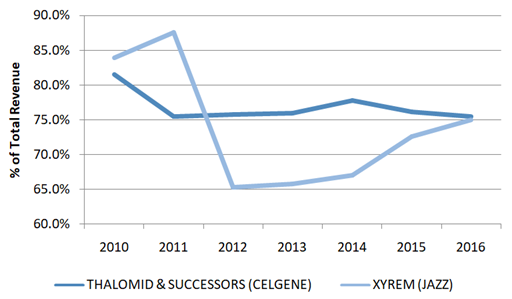
Celgene’s Thalomid (and its successors Revlimid and Pomalyst) and Jazz’s XYREM are examples of drugs that have used the REMS program to extend pricing power. Both companies have developed billion dollar business through the exclusivity of the REMS program.
Figure 6: More than 65% of Celgene & Jazz’s Revenues Are Generated From Compounds Under REMS Program
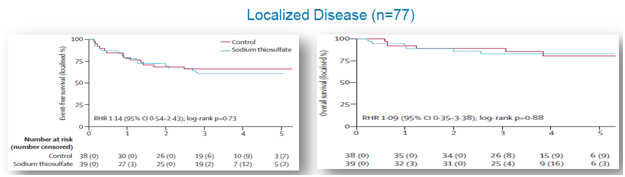
STS Was Safe to Administer In Localized Cancers
For localized cancer, EFS for the active group was 60% compared to 66%for the control group (p=0.73), while OS for the active group was 83% compared to 89% for the controlgroup (p=0.88), shown below.
Figure 7: In localized cancers, STS arm did not see change in overall survival
The main takeaways from COG ACCL0431:
- STS works to protect against cisplatin-induced hearing loss in children, especially for those < 5 years old
- STS intervenes with chemotherapy toxicity in disseminated cancers, but STS is safe to administer in localized tumors. The latter further validated by interim SIOPEL 6 data, discussed in section below.
Second Phase III Study(SIOPEL 6): STS did not intervene with the anti-tumor activity of the platinum-based chemotherapy [6] and efficacy data was in line with prior studies.
The second Phase III study, SIOPEL 6, enrolled children between 1 and 18 years of age with standard risk hepatoblastoma (childhood liver cancer). It is important to note that high-risk hepatoblastoma patients were excluded from enrollment. This indicates that all patients had characteristics of a “local disease”, which the first Phase III study showed was safe. Patients were randomized to receive cisplatin according to their standard regimen + STS (active arm) and just cisplatin according to standard regimen (control arm).
Data showed that it was safe to deliver STS as it did not intervene with Cisplatin's anti-tumor activity. Additionally, efficacy numbers were in line with the first Phase 3 study ("COG ACCL0431").
Efficacy: Among the 99 evaluable patients, hearing loss occurred in 30/45=67% treated with Cisplatin (Cis) alone and in 20/54=37.0% treated with Cis+STS (p=0.0033).

Safety: The combination of Cis+STS was well tolerated and showed it did not interfere with cisplatin’s anti-tumor effects.
- With a follow up of 52 months, 3yr EFS is Cis 78.8% and Cis+STS 82.1%;
- 3yr OS is Cis 92.3% and Cis+STS 98.2%.
The main takeaways from SIOPEL 6 interim:
- Validates that STS is safe to deliver as it is not intervening with cisplatin’s anti-tumor activity in a localized cancer indication
- Validates that STS protects against hearing loss in children under 5 years old
Bottom Line: In our view, positive SIOPEL 6 data was a huge de-risking event for Fennec. The data proved that STS worked and was safe to administer in a small population (localized hepatoblastoma). We think this data is enough to get Fennec to push for FDA approval and then target off label use in children under 5 years of age with localized cancers.
We Value Fennec at $21/Share If Company Markets STS Themselves
Using the American Cancer Society’s Cancer in Children & Adolescents Report as a reference, we estimate there are approximately 2300 children and adolescents in the US with localized cancer.Similarly, International Agency for Research on Cancer was used to determine there are 3100 children and adolescents in Europe. We estimate that roughly 1900 children in the US & Europe are diagnosed with localized cancer and are under the age of 5, the target population that STS has proven to be most effective and Fennec’s immediate target market.
Fennec plans to file a NDA by mid-2018. We model STS to hit the market by mid 2019 and peak sales to reach $76M in 2025. Price of STS was assumed to be $50,000, on the low end of alternative hearing aid costs, which run $50,000- $70,000.
Figure 8: Discounted Cash Flow method yields a price of $20.61/share, assuming a 10x EBITDA multiple and 25% discount rate
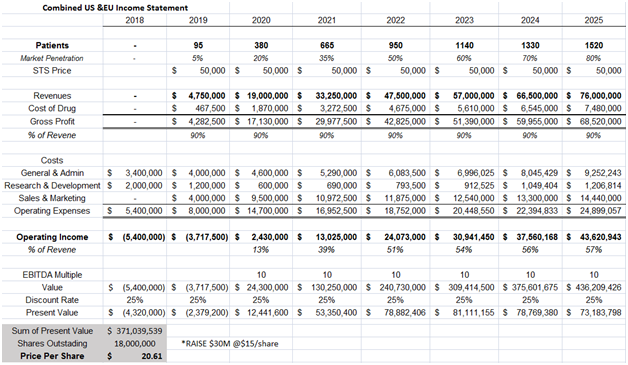
In our model above we assume:
- STS is given to 80% of our target market population of localized cancers and children aged under 5 years of age. No children over 5 years old and no disseminated cancers are subscribed STS
- STS gross margin to be 90% since the compound has been approved for over 60 years and is not expensive to produce
- Operating margins to be in the high 50% range. Fennec has been testing STS in Phase III trials for a decade, so most pediatricians are aware of STS which should taper marketing spend.
- 25% discount rate is used to take into consideration the approval and commercialization risk associated with STS
- We assume Fennec raises $30M at $15/share to fund STS commercialization efforts
We believe the above assumptions are on the conservative end and that a higher valuation can be argued for.
With Positive Interim Data, Fennec is Obvious Acquisition Target
We believe Fennec is positioned to be bought out. The company is very tightly held as 85% of the shares outstanding are owned by insiders and funds, shown below.
Figure 9: Fennec shareholders include healthcare funds venBio, Acuta and Essetifin, to name a few
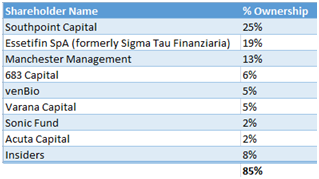
Not only is such a roster of funds rare to find in a small biotech, but with Fennec being a thinly traded stock, the only realistic exit for these funds is a takeout. Further pushing the M&A angle, Fennec has more than $130M in income tax loss carry forward which adds to the appeal as an acquisition target.
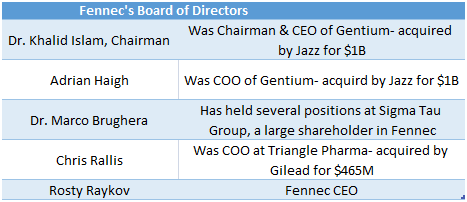
Fennec Board Made Up of Members with Prior M&A Experience
Fennec boasts a very impressive board made up of directors who have had successful exits in rare diseases. If Phase III data lines up, Fennec has a board with enough industry contacts to put the company on the M&A map and set up meetings with Jazz Pharmaceuticals or Sigma Tau’s pharmaceutical arm, Leadiant Bio.
Figure 10: Fennec’s board constructed with executives from biotech buyouts
Acquirer Would Be Drawn to Fennec’s Economic Moat
Figure 11: In the last four years, orphan drug companies have been acquired at multiples between 1.6x-5.2x peak projected sales.
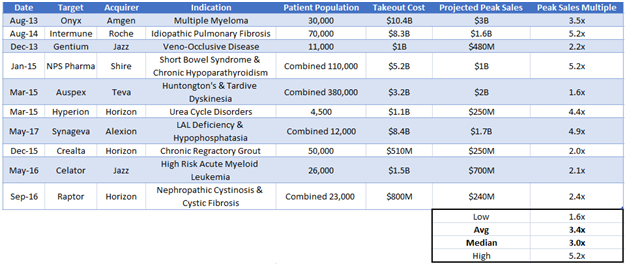
We believe Fennec will command a multiple on the high end of the range where other rare disease companies have been bought out. We calculate Fennec’s takeout value to be approximately $350M, a 4.5x peak sales multiple given our $76M peak sales forecast. This equates to ~$21/share.
Our calculations are supported by Fennec’s strong barriers to entry. The FDA will require any competing product to prove its efficacy in pediatric patients over a long term period. For Fennec, getting to an NDA was a 10 year Phase III study. We expect the FDA will require similar decade long data from other compounds interested in treating chemo-induced hearing loss. Additionally, the pricing power from a REMS program, discussed above, will create an exclusive economic moat for Fennec longer than the 7 & 10 years provided by Orphan Drug Designation and European Market Exclusivity, respectively.
Fennec’s Current Opportunity is a Result of Legacy Issues, Which We Believe Are No Longer Applicable
Fennec is mispriced due to legacy issues and lack of exposure. The company has 2 prior failed drugs (in breast cancer and melanoma) and until recently, was trading on the TSX/OTC markets with little liquidity. The STS Phase III studies make a very compelling case for the company to receive approval and until recently, no prevalent investors knew of the name. These issues are of the past, but have created the current opportunity in Fennec.
One or more of PropThink's contributors are long FENC
SOURCES:
[1] https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet#r3
[2]http://ascopubs.org/doi/full/10.1200/jco.2011.39.1110
[3] https://www.ncbi.nlm.nih.gov/pubmed/15519518
[4] http://www.healthyhearing.com/help/hearing-aids/priceshttp://www.asha.org/public/hearing/Cochlear-Implant-Quick-Facts/
[5] http://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(16)30625-8/abstract
[6] http://meetinglibrary.asco.org/record/125271/abstract
Access This Content Now
Sign Up Now!




