Sarepta Therapeutics (SRPT) has come a long way since late 2013, when lead candidate eteplirsen’s approval looked like it might be years out.
The company will file a New Drug Application for eteplirsen in Duchenne Muscular Dystrophy by the end of 2014 and has laid out an aggressive development plan for confirmatory eteplirsen trials, and studies of two new exon-skipping therapies also to begin by year-end. SRPT priced 2.65 million shares in a secondary offering in April, days after conveying to investors a plan to get the drug in front of the FDA for an approval decision.
When SRPT jumped on news of an accelerated approval on April 21, we were optimistic that investors could scale back in over the next few months. Our recommendation to book some gains around $38 (more than a 150% return from January), has proved prescient. SRPT now trades at $34 as demand for small-cap biotech vacillates.
The FDA is open to a New Drug Application by year-end, but indicated that more efficacy and safety data would help in the review process. Sarepta has two options: submit more dystrophin data as a surrogate endpoint, and/or generate additional 6-Minute Walk Test data. The company will pursue both. Sarepta says the FDA will be flexible about accepting more data after the NDA is submitted, during the review process in the first half of next year.
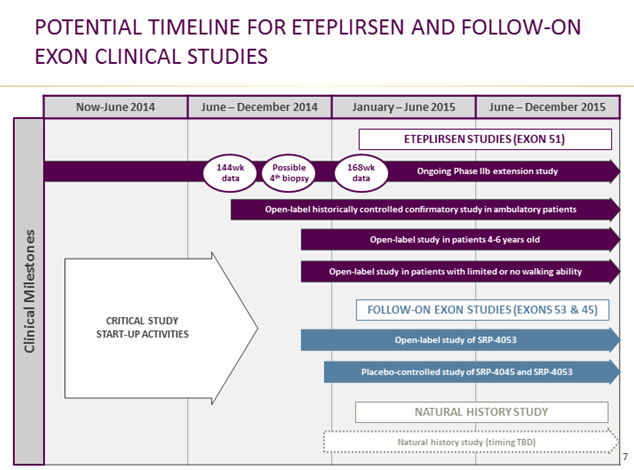
Before filing, Sarepta will have 144 week data from the ongoing Study 202 this summer (expects to present results at World Muscle Society Congress in early October), and will provide 168-week data early in 2015. The company is considering a 4th muscle biopsy for boys currently receiving eteplirsen to enhance the existing dataset that goes to the FDA, but made clear that they’ll take an active roll in guiding the FDA through this dystrophin assessment/collection process. The FDA is getting uncharacteristically involved in understanding dystrophin as a surrogate, encouraging for investors.
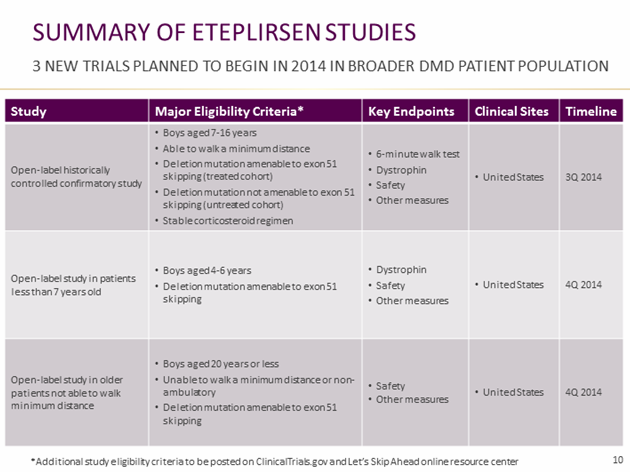
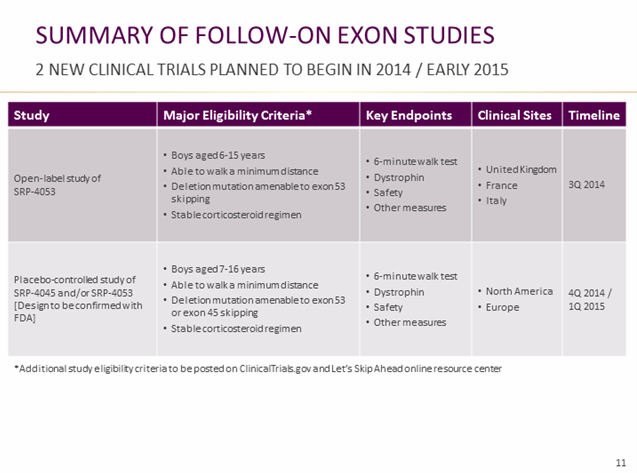
On Tuesday, Sarepta presented an update on its clinical development program via webcast. The company plans to begin 5 new studies before the end of 2014.
1) The first study in the slide above is the eteplirsen confirmatory study. If the FDA approves eteplirsen through an accelerated approval, this is the study that will be used to confirm the drug’s efficacy and safety while eteplirsen is on the market. The study will enroll 60-80 ambulatory patients, age 7 to 16 years, who can walk a minimum distance (6MWT). The trial will enroll a parallel untreated cohort of approximately 80 patients who are not amenable to exon-51 skipping. The untreated cohort may include patients amenable to skipping of exons -45 and -53, Sarepta’s next two therapeutic targets, in order for the company to begin collecting data on these patients.
2) The second and third studies listed above will test eteplirsen in exon-51 amenable younger DMD boys (aged 4-6); and 3) in older patients (age 16-20) who are unable to walk a minimum distance. These trials will evaluate eteplirsen’s safety, and dystrophin as a surrogate, in 20 patients each.
4) A 24-patient open-label study of Sarepta’s exon-53-skipping therapy, SRP-4053, will begin in Europe later this year. The company also expects to begin dosing (5) in a domestic placebo-controlled trial of SRP-4045 and/or SRP-5053 by early next year. The study will incorporate 6MWT and dystrophin endpoints.
6) Sarepta has given limited information on a natural history study to begin in 2015.
144-week data from Study 202 are due around mid-year, possibly as early as June/July.
One or more of PropThink’s contributors are long SRPT.