Retrophin Inc (RTRX) shares dropped 12% on Friday morning with the release of results from a single Pantothenate kinase-associated neurodegeneration (PKAN) patient taking Retrophin’s RE-024. The stock staged an all-day rally, however, and closed in the green as investors digested the positive efficacy trends, despite early toxicity, seen in the single patient.
The market seems to agree with Retrophin, which called the results “inconclusive” in the SEC filing – on Monday, RTRX is nearly flat from Thursday’s close.
Retrophin issued an 8K on Friday morning outlining outcomes for a single patient receiving RE-024 over the course of one month. The patient first received-024 on May 21, and on June 5 developed elevated liver enzymes (1-2x the upper limit of normal). Dosing was discontinued. The patient’s AST and ALT levels had dropped at the next follow-up visit on June 11, and RE-024 was restarted at a reduced dose. The patient has maintained normal levels since and was on treatment at June 25.
The market punished RTRX initially for -024’s apparent toxicity, but a deeper reading of the results demonstrate some signs of early efficacy. From the filing:
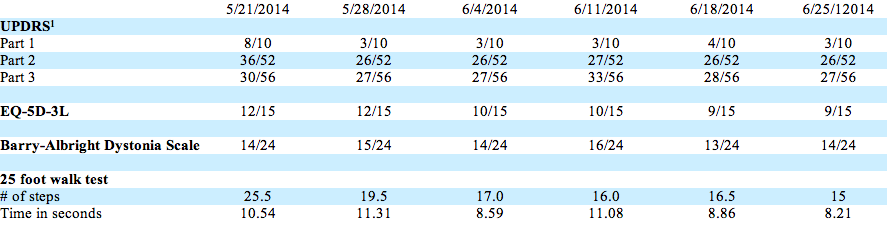
Over the course of 28 days on drug, improvements were seen on Parts 1-3 of the Unified Parkinson’s Disease Rating Scale (UPDRS); EuroQol Group’s EQ-5D-3L (a broad health outcome evaluation); and the 25-foot walk test. The patient showed no change on the Barry-Albright Dystonia Scale. Emory Redd, of Schenley Park Advisors, created a handy visualization of the data.
These results come from a single patient on -024 for less than one month, easily influenced by subject-expectancy. Although early outcomes are encouraging, over-analyzing the data may prove a dangerous errand.
One or more of PropThink’s contributors are long RTRX.