Orexigen Therapeutics (OREX) ripped higher on Tuesday with news that a new – and profoundly important – patent has been issued for Contrave, Orexigen’s approved weight loss drug. It’s not the patent (‘371 Patent) that has investors excited; it’s what the patent covers. Contrave appears to not only help patients lose weight but may also reduce their risk of a major cardiovascular event, like stroke or heart attack. It’s a bit early, but the data are moving in the right direction.
Contrave is approved as an adjunct to a reduced-calorie diet for weight management in adults with an initial body mass index of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight). The FDA agreed to approve Contrave based on a number of trials measuring the drug’s ability to improve weight loss relative to placebo. But, the current label says that Contrave’s effect on cardiovascular/survival outcomes (stroke, heart attack, death, etc) has not been established. The FDA required that Orexigen run a large, controlled trial, the 8,910-patient LIGHT study, measuring Contrave’s effect on these Major Adverse Cardiovascular Events (MACE) compared to placebo.
The new ‘371 Patent and provisional patent applications incorporate data from an interim analysis of the LIGHT Study, which was conducted when 25% (94) of the expected MACE had occurred, and was included in Orexigen’s New Drug Application to the FDA. The pre-planned interim analysis was designed to exclude a doubling of CV risk compared to placebo. This is the first time investors are seeing this data. Here’s what has OREX up 45% on Tuesday:
Contrave significantly reduced the risk of MACE (including Myocardial Infarction, Stroke, or CV Death) compared to placebo at the 25% interim analysis. Below is a Kaplan Meier analysis for time to first MACE in the Intent-to-Treat population based on the 25% interim analysis.
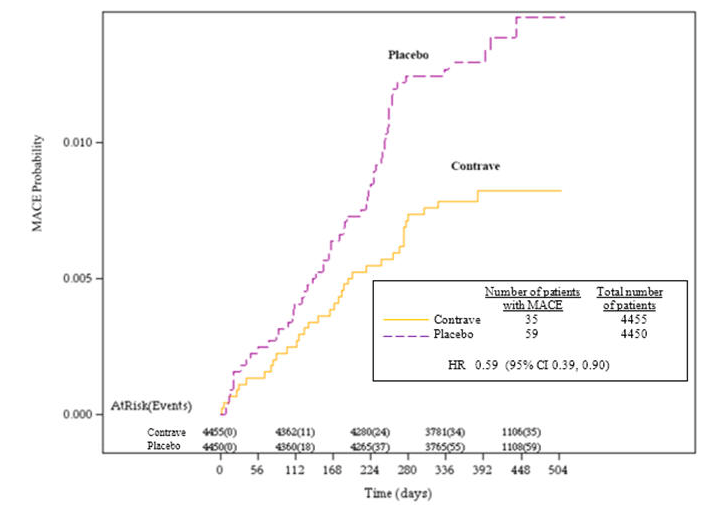 Though this is just the first interim look at LIGHT, Contrave’s impact on cardiovascular events is clearly trending in the right direction. Orexigen points out that a larger number of MACE are required to precisely determine the effect of Contrave on CV outcomes.
Though this is just the first interim look at LIGHT, Contrave’s impact on cardiovascular events is clearly trending in the right direction. Orexigen points out that a larger number of MACE are required to precisely determine the effect of Contrave on CV outcomes.
Orexigen’s partner for North America, Takeda, launched Contrave in the U.S. in October of last year. Net sales for the fourth quarter were $6.5 million (Orexigen earned $1.3 million in royalties). With Tuesday’s data, the apparent gap in efficacy between Contrave and competing products from Arena Pharmaceuticals (ARNA) and Vivus (VVUS) is widening.
One or more of PropThink’s contributors are long OREX.





