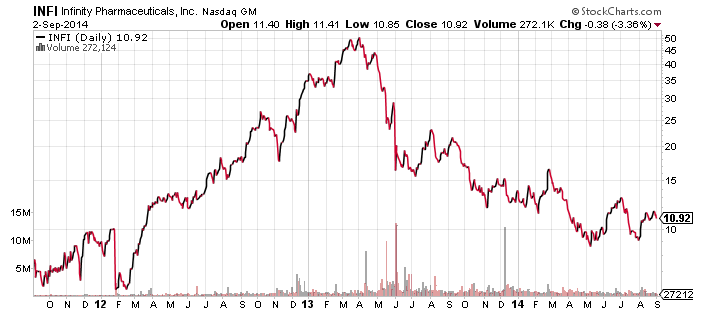
Infinity Pharmaceuticals (INFI) and AbbVie Inc. (ABBV) have entered into a major partnership to develop and commercialize duvelisib (IPI-145), Infinity’s oral phosphoinositide-3-kinase (PI3K) inhibitor. PI3K inhibitors were the cat’s meow in 2013, and Infinity’s was briefly a Wall St. darling. But with the approvals of Gilead Science’ (GILD) Zydeliq, also a PI3K inhibitor, and Pharmacyclics’ (PCYC) Imbruvica, most small companies that received attention for their PI3K programs had been left for dead. This chart says it all:
Infinity, and likely other PI3K players, are certainly back in vogue with this deal; the stock is up 45% in early trading on Wednesday.
AbbVie is paying Infinity $275 million upfront and is on the hook for up to $530 million in additional milestone payments, including $405 million through the first commercial sale of duvelisib. The companies will split commercialization expenses and profits in the U.S. AbbVie will be responsible for commercializing duvelisib ex-U.S, and Infinity is eligible to receive tiered double-digit royalties on net product sales.
Infinity is already testing duvelisib in one phase 3 chronic lymphocytic leukemia (CLL) study, dubbed DUO, and in a phase 2 study in patients with indolent non-Hodgkin lymphoma (iNHL), DYNAMO.