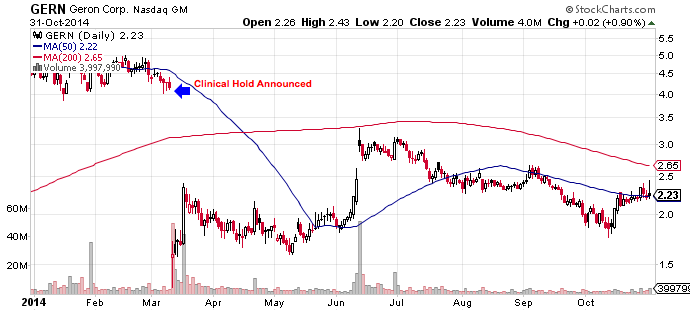
The FDA has removed a clinical hold prohibiting Geron Corp (GERN) from dosing new patients with its investigational cancer drug imetelstat, sending the company’s stock higher by 35% to $2.90 on Monday.
This March Geron received notice from the FDA that its Investigational New Drug application for imetelstat had been placed on full clinical hold after low-grade liver function test (LFT) abnormalities were observed in some essential thrombocythemia (ET) and polycythemia vera (PV) patients receiving the drug. With the lift of the full clinical hold, a multi-center phase 2 study of imetelstat in myelofibrosis will begin in the first half of 2015. The company won’t conduct any further studies in either indication and will focus instead on high-risk myeloid malignancies, like myelofibrosis.
To lift the hold, Geron followed patients who experienced LFT abnormalities until resolution to normal or baseline. In the ET trial, abnormalities resolved to normal or baseline in 14 of 18 follow-up patients, while 3 of the remaining 4 showed improvement in LFT abnormalities. One patient still had unresolved LFT abnormalities. The company continues to follow two of the remaining four ET patients. In a discontinued multiple myeloma trial, LFT abnormalities resolved to normal or baseline in all nine follow-up patients. The company said that no new emergent hepatic adverse events were reported during follow-up. The company also submitted animal data from previous toxicology studies.
Geron had $142.5 million in cash and investments at September 30, or $0.90 per share.