Drug development under the eye of the U.S. Food & Drug Administration (FDA) is a notoriously long and fraught process, spanning an average of 11 years where only 8% of proposed products reach approval.[1] Aware of the limitations this presents for both physicians and patients, the FDA recognizes four programs aimed at facilitating and expediting the development of new drugs that address unmet needs for serious or life threatening diseases. These designations present an opportunity for drug developers – and investors – to take a speedier path to drug approval.
All of the expedited programs aim to ensure that new medicines for serious conditions reach patients as soon as the FDA can conclude that the benefits justify the risks. For this reason, these designations are only available to drug candidates that are intended to treat a serious condition with an unmet medical need. For the purposes of all four expedited programs, a serious disease is defined as:
…a disease or condition associated with morbidity that has substantial impact on day-to-day functioning. Short-lived and self-limiting morbidity will usually not be sufficient, but the morbidity need not be irreversible if it is persistent or recurrent. Whether a disease or condition is a serious is a matter of clinical judgment, based on its impact on such factors as survival, day-to-day functioning, or the likelihood that the disease, if left untreated, will progress from a less severe condition to a more serious one.[2]
Briefly, recall that the FDA’s current review process usually takes 12 months. First, a New Drug Application (NDA) is submitted to the agency. The FDA has 60 days (2 months) to review and then accept the application for filing. NDA sponsors (usually the pharmaceutical company) will generally announce both events. The FDA’s review of the NDA then takes 10 months.
It’s critical for investors to understand that NONE of the designations here should be considered validation of a company’s technology or asset. These are all conferred based on the size and need of the intended population. This is a common mistake among novice healthcare investors: assuming that a Breakthrough Designation or Priority Review means the FDA likes or wants to approve a drug. This is simply not the case.
Types of Designations:
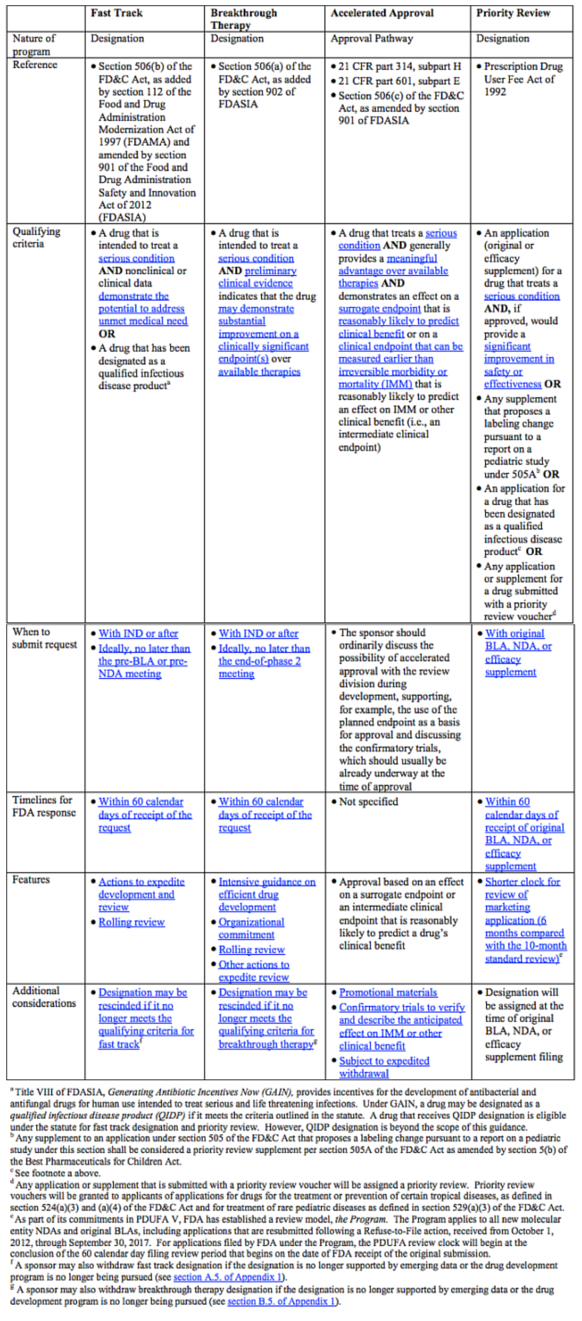
The FDA has created four expedited programs: Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review.[3] We will discuss each in turn, as well as their individual advantages. For review in a glance, the chart at the bottom of this article is a great quick-reference.
Fast Track Designation
The Fast Track designation is available for drug candidates that are “intended, whether alone or in combination with one or more other drugs, for the treatment of a serious or life threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition.”
Furthermore, the drug candidate must demonstrate – clinically or non clinically – the potential to address an unmet need. That is, this can be done early in development by showing the potential, often via mechanistic rationale or pharmacological data. Alternatively, later in development, clinical data can be used.
The designation essentially allows for more frequent and early interactions and consultations with the FDA, to discuss efficient trial design, dose-response concerns, and extent of safety data required. Fast Track assets can obtain a rolling review (which allows the FDA to review completed sections of a New Drug Application rather than waiting until the entire application is complete for review).
The application can be done any time at or after submitting an Investigational New Drug Application (IND), though no later than the pre-New Drug Application (pre-NDA) or pre-Biologics Licence Application (pre-BLA). The FDA is scheduled to respond to the application within 60 calendar days of receiving the request.
For reference and to support the assertion that these designations are NOT major validation from the FDA, a whopping 75% of all Fast Track Designation requests between 1998 and 2006 were granted. In more recent years, that number has been as high as 80%. That’s not a tremendously high bar for success.
Breakthrough Therapy Designation
This designation is available to drugs that are intended to treat a serious condition and have preliminary clinical evidence indicating that the drug may allow substantial improvement on a clinically significant endpoint over available therapies. The designation allows for all the Fast Track program features, as well as more intensive FDA guidance on an efficient drug development program. It also benefits from rolling and Priority Review.
Similar to Fast Track, Breakthrough Therapy applications can be done at the time an Investigational New Drug Application (IND) is submitted or after, though no later than end-of-phase 2 meetings. The FDA is scheduled to respond to a request within 60 calendar days.
Accelerated Approval Pathway
Accelerated Approval is available to drugs that treat a serious condition and provide a meaningful advantage over available therapies. Additionally, the drug candidate must have demonstrated either (1) an effect on a surrogate endpoint (marker) that is reasonably likely to predict a clinical benefit; or (2) a clinical benefit that is reasonably likely to predict an effect on irreversible morbidity, mortality or other clinical benefit.
This pathway is mainly used in cases where the course of the disease is long, thus requiring an extended period of time to measure intended clinical benefits (for example cancers, where tumor growth can be rapidly assessed but effect on survival or morbidity require lengthy trials). It is also commonly seen in acute disease settings that require large studies as the targeted clinical events occur rarely or slowly.
Unlike Fast track and Breakthrough designations, there is no clear indicated time for requesting an Accelerated Approval. Instead, the sponsor discusses the designation with the FDA review division during development.
The pathway allows for a drug candidate to receive approval based on surrogate or intermediate clinical endpoints, rather than final clinical endpoints. However, the sponsor is generally required to conduct post-marketing trials to verify and describe the drug’s clinical benefit, as the FDA reserves the right to an expedited withdrawal if followup trials fail to verify the expected benefit.
Priority Review
This designation is aimed at drugs that, if approved, would provide significant improvements in safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition when compared to existing therapies. The application can be filed along with the New Drug Application (NDA) or the Biologics Licence Application (BLA).
By achieving this designation, the FDA expedites the review of a marketing application, shortening the review to 6 months (the norm is 10 months under a standard review)
Note: Overlapping Designations
These designations do not always work independently. Drugs given Fast Track or Breakthrough Designations can be eligible for accelerated approval. Similarly, all designations can also apply for Priority Review if eligible.
Comparison of FDA’s Expedited Programs for Serious Conditions[4]
[1] An Overview of the Drug Development Process, http://www.fdareview.org/approval_process.shtml
[2] 21 CFR 312.300(b)(1).
[3] For a comparison of all FDA expedited programs, see table in Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics, at pages 11 and 12. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm358301.pdf
[4] Table originally from Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics, at pages 11 and 12. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm358301.pdf