ONCS has two ipcoming catalysts with PICES data in November for metastatic melanoma and OMS-140 data for TNBC in December. Prior data (discussed below) leads us to believe the hurdle for both catalysts is relatively low.
Here's what we found to support Oncosec's upcoming data readouts:
Checkpoint drugs (Yervoy, Opdivo and Keytruda) work best in tumors that are highly infiltrated with cancer fighting immune cells (known as “hot” or “immunogenic” tumors). The majority of tumors, however, are "cold" or “non-immunogenic”, meaning they lack the immune cells to fight the cancer, even if it is detected.
This is where combination therapies (checkpoint inhibitors + immunomodulators or oncolytic viruses) are expected to breakthrough because they will essentially convert cold tumors into hot tumors. They do this by driving more T cells into the tumors, setting the stage for checkpoint drugs to be more effective.
Oncosec’s thesis: ImmunoPulse delivery device plus IL-12 (called “Tavo”) will convert cold tumors into hot ones, thus allowing checkpoint drugs to work in patients, who would otherwise be unresponsive.
Studies in Metastatic Melanoma
OMS-100: Tavo monotherapy treatment for metastatic melanoma
Data Expected: Nov 29 – Dec 1 @ Melanoma Bridge Conference
OMS-100 enrolled 51 metastatic melanoma patients into 3 cohorts to receive Tavo. Data was initially presented at SITC 2017 and showed that in the cohort with the more frequent dosing, 10/28 patients (36%) experienced an objective response rate (ORR). The goal of this study was to determine the optimal dose for combination with a checkpoint inhibitor (which paved the way for PICES study, below).
What to expect: The full data will be presented at Melanoma Bridge, but we don’t believe it will have any material updates. The 36% ORR suggests that Tavo monotherapy activates the immune system, which may enhance response to checkpoint inhibitors.
OMS-102: Tavo combination with Keytruda in predicted checkpoint non-responders
Patients in this study were predicted to be refractory to checkpoint therapy through a biomarker assay (not actual non-responders). Only 11/23 patients had a prior checkpoint therapy.
The Tavo+Keytruda combination produced an ORR in 9/21 patients (43%). Importantly, all responses were durable beyond 12 months. mPFS not reached, but 57% of patients were continuing to respond to treatment at 15 mos.

The data above suggests that Tavo+Keytruda combination could be an effective therapy in patients unlikely to respond to checkpoints.
Oncosec is running PICES trial to test actual patients that have not responded to checkpoint inhibitors.
PICES/KEYNOTE-695: Tavo combination w/ Keytuda in checkpoint non-responders
Data Expected: November 7-11 @ SITC
Unlike OMS-102, which used biomarkers to predict non-responders, PICES will enroll up to 80 patients that have failed all prior therapies and have not responded to at least 4 prior doses of checkpoint therapies. These patients will likely have to go back to chemo, which has about a 10% response rate at this stage.
The PICES trial was originally broken down into two stages. Stage 1 enrolled 23 patients and needed to show response in at least 4/23 (17%) to commence Stage 2. This was recently changed to increase the number of patients enrolled from 48 to 80 patients to better position Oncosec for Accelerated Approval with FDA (discussed more below).
Oncosec will report top line data at SITC in November.
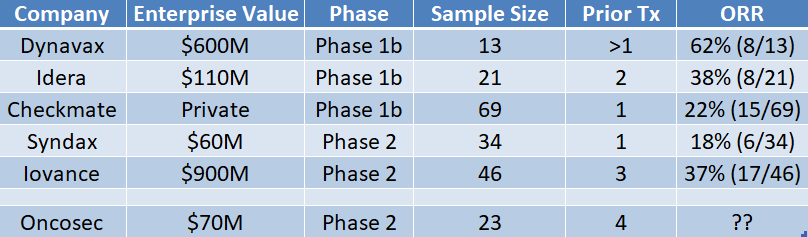
ONCS Has to Report At Least 6/23 (26%) Responders
We believe the company will have to show at least 6/23 (26%) responses to be clinically significant and competitive with other therapies in development (see below).
Oncosec’s data will need to show that the responses are durable and the safety profile is clean.
$3/share With Good Readout
With a 26% or better response rate, we believe ONCS could warrant a valuation that is at least double what the company currently trades at. Keep in mind, this study is enrolling extremely sick patients that have failed several (4+) checkpoint therapies. A high response rate would bode well if Oncosec decided to test the candidate on less sick patients.
If successful, Oncosec plans to use PICES data to apply for Accelerated Approval (in late 2019- early 2020) for unresectable metastatic melanoma patients who have progressed or are progressing on an approved anti-PD-1 therapy. The company has a well defined definition of what a checkpoint failure is, which is needed for accelerated approval discussions.
ONCS Has Defined Checkpoint Failure And Enrolling Additional Patients for Accelerated Approval
Earlier this month, the FDA required Iovance (IOVA) to run a 4th arm (n=80-100) in their Phase 2 advanced melanoma study. We believe that this additional arm was required by the FDA because Iovance wanted to apply for accelerated approval, but had an inconsistent definition for what a checkpoint failure was.
With positive data, we think Oncosec is on the right path to Accelerated Approval. Iovance’s 4th arm with 80-100 patients is a good measure for Oncosec’s increased enrollment size of 80 patients.
Bottom line: We believe that Oncosec will have to show at least 6/23 (26%) responders in “Stage 1” of the PICES study to be clinically significant and competitive with other therapies in development. Oncosec’s prior studies in melanoma (35%+ response rate) show that Tavo activates the immune system and Tavo+Keytruda combination could be an effective therapy in patients unlikely to respond to checkpoints. We believe 26% or higher is achievable.
Studies in Triple Negative Breast Cancer
OMS-140: Tavo monotherapy biomarket study in TNBC
Data Expected: Dec 4 – Dec 8 @ San Antonio Breast Symposium
Oncosec’s tavo monotherapy is a small study with 10 advanced or metastatic TNBC patients receiving one cycle of tavo. The main purpose of the study was to test immunology, however the clinician (Dr. Melinda Telli) gave nivolumab as an immediate next therapy to to 2/5 patients and saw “clinically meaningful objective responses” in these 2 patients. As a result of being a clinical observation, this “combo” piece of the study was not part of the protocol and no measurements were taken.
ONCS will present top line data in all 10 patients in December. Since the combination treatment was anecdotal, it is unclear if the remaining patients will receive combination with nivolumab. This data, although early, encouraged the company and Merck to initiate OMS-141 trial (tavo+keytruda) in late stage TNBC. Data for OMS-141 is expected in 2019.
Early stage studies of other second line treatments in TNBC have shown response rates under 20%. Immunomedics (IMMU) has grown to a $4B company after reporting a 30% ORR in metastatic TNBC patients that have had 3 prior lines of treatment.
Companies that can show double digit response rate in metastatic TNBC are rare and valued as such. Oncosec has very early data that potentially support a good response rate.

What to Expect with OMS-140: ONCS has reported that 2 patients (out of first 5) experienced clinically meaningful objective responses. Even if we assume that none of the second group of 5 patients show a response, 20% (2/10) would warrant further research.
Keep in mind that Phase 1 OMS-140 data is extremely early and was anecdotal (clinical observation outside study scope). It is not clear whether the second group of 5 patients will also receive nivolumab as an immediate therapy following tavo. If patients do receive combo and responses are again shown, that would be an achievement for ONCS (warrant a share price north of $3/share).
Bullish indicator: Merck saw enough in tavo to initiate a combination with Keytruda in TNBC (OMS-141 trial).
ONCS has two big upcoming catalysts with PICES data in November for metastatic melanoma and OMS-140 data for TNBC in December. We believe the hurdle for both catalysts is relatively low. At $70M enterprise value, ONCS presents a favorable risk/reward (we believe about 3:1).
One or more of PropThink's contributors are long ONCS.
Access This Content Now
Sign Up Now!




