BioDelivery Sciences (BDSI) and partner Endo Health Solutions (ENDP) announced on the 23rd that the second of three phase 3 trials testing one of its lead drug candidates, BEMA buprenorphine, as a treatment for chronic pain has been complete with positive results. The stock climbed from a close of $6.25 on the 23rd to a high of $9.49 on Friday the 24th before coming under pressure in the trading sessions following. In early December, PropThink suggested being long the stock ahead of these pivotal results, results that are key to the long thesis due to a phase 3 failure in 2011, which we discuss below. With three meaningful catalysts in the first half of 2014 and an important overhang out of the way, BDSI is an attractive holding into mid-year, particularly as the stock bases around $8.00. We like the idea of buying BDSI in the low $8.00-range as a trade into mid-year, understanding that an equity financing around the corner may not be such a bad thing
Phase 3 Opioid–Naïve Results Remove a Key Hurdle
BEMA buprenorphine is being developed for the treatment of moderate to severe chronic pain in patients requiring around-the-clock opioid therapy for an extended period of time in both patients who are opioid-naive or opioid-experienced.
The now-complete trial, BUP-308, was a phase 3 efficacy study of BEMA buprenorphine in opioid-naïve subjects. Details from the study were sparse, but the trial successfully met its primary efficacy endpoint in demonstrating that BEMA buprenorphine resulted in improved chronic pain relief compared to placebo (p<0.005). Endo Health Solutions, BDSI’s partner for the candidate, said that secondary endpoints were supportive of efficacy compared to placebo as well. The most commonly reported adverse events in patients treated with buprenorphine vs. placebo were nausea (10% vs. 8%), vomiting (4% vs. 2%) and constipation (4% vs. 2%).
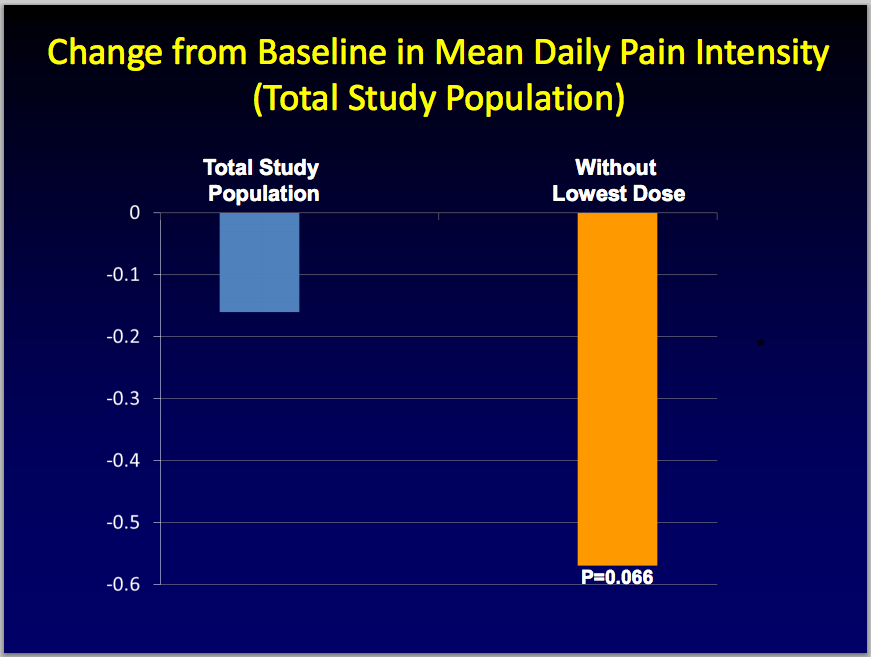
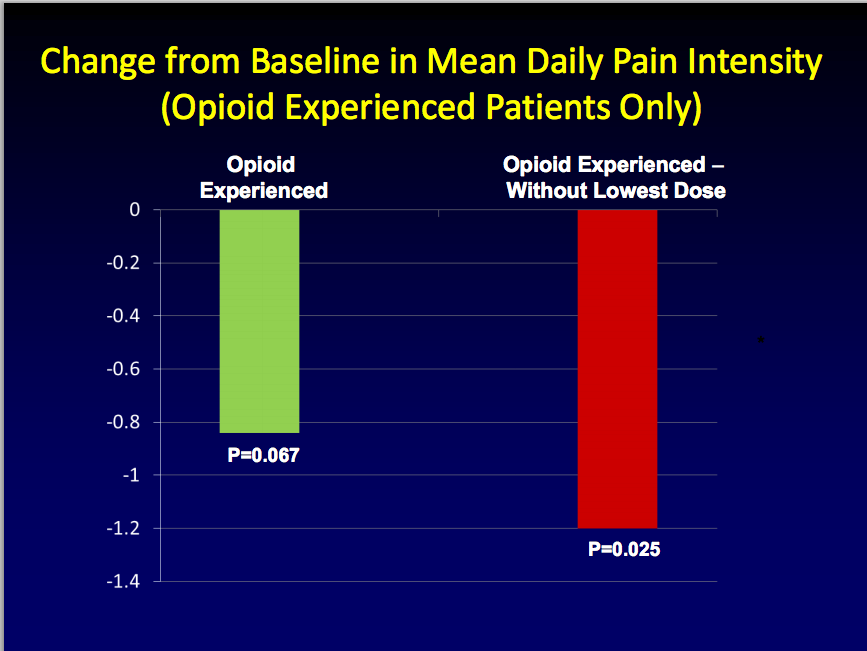
Success in the opioid-naïve population is important for investors because opioid-naive patients confounded the results of a previous phase 3 study that combined the two groups, -naïve and -experienced patients. In early 2011, BioDelivery announced the results from this combined study. The primary endpoint of the trial, BUP-301, was the overall pain intensity difference between BEMA buprenorphine and placebo. BEMA buprenorphine failed to demonstrate statistically significant efficacy compared to placebo, which the company attributed to a high response rate in the opioid-naïve segment of the placebo arm, particularly at the lowest, starting dose of BEMA buprenorphine. Upon further analysis, it appeared that eliminating the lowest dose group from the total population in the trial resulted in a treatment difference that was nearly statistically significant (p=0.066).
In addition, in the sub-population of opioid-experienced patients, the treatment difference was also nearly statistically significant (p=0.067). Backing out the lowest dose in opioid-experienced patients resulted in statistical significance (p=.025)
BDSI and Endo (which signed on as commercialization partner for BEMA buprenorphine months after these results) designed a phase 3 program around these two patient subgroups – trial BUP-307 in opioid-experienced patients, and BUP-308 in opioid-naïve patients.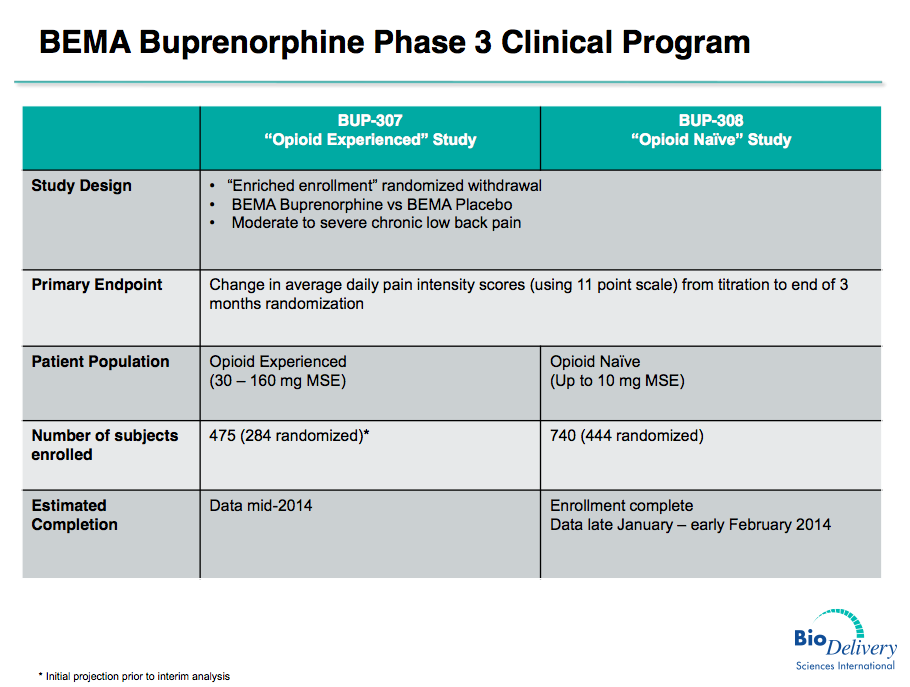
Given the confounding results of BUP-301, it was this latest trial, BUP-308 testing BEMA buprenorphine in opioid-naïve patients, that was the bigger unknown. With success, investors can breath a sigh of relief and should view BUP-307 and the asset as a whole as significantly more de-risked.

Already a Premium user? Sign In