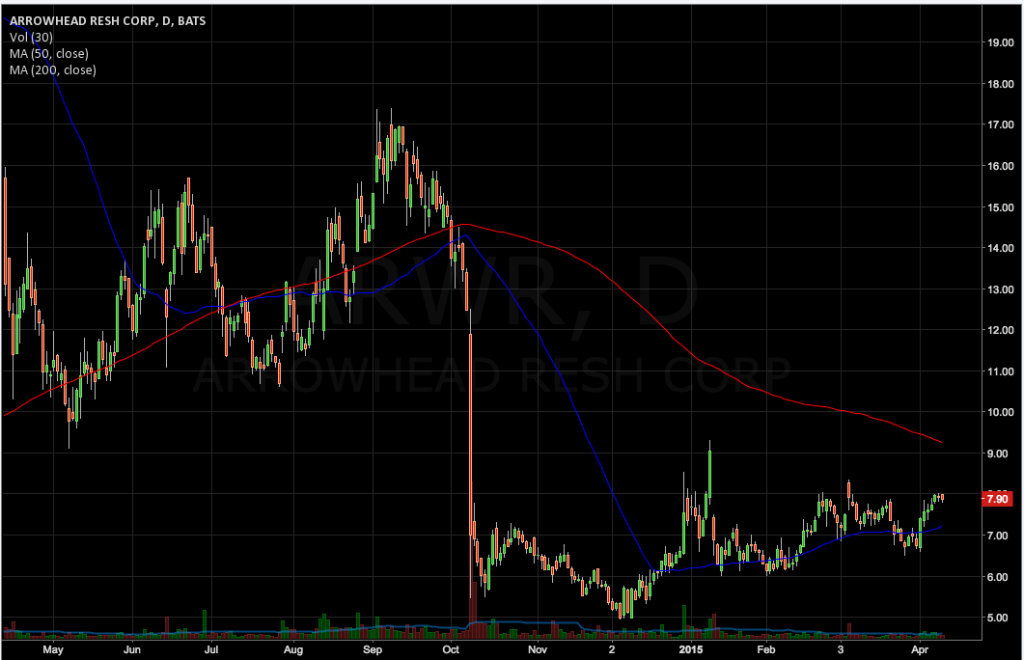
A week after the FDA modified a similar restriction on Tekmira Pharmaceuticals (TKMR), the federal agency has cleared Arrowhead Research (ARWR) to proceed with a multiple-dose Phase 2b clinical study of ARC-520, its candidate for the treatment of chronic hepatitis B infection. A single-dose study of the drug is currently ongoing, with results expected by the end of the second quarter.
The clinical study, to be called Heparc-2004, is a multicenter, randomized, double-blind, placebo-controlled, multi-dose study of ARC-520 in patients with chronic HBV infection who are maintained on standard antiviral therapies, entecavir or tenofovir. Eight patients in the study will receive 1 mg/kg of ARC-520, 4 patients will receive placebo, and all will receive 3 total, administered once every 4 weeks. Patients will be followed through Day 147.
Arrowhead is currently testing single doses of ARC-520 in chronic HBV patients and will announce top-line results from two remaining arms of the study, a 3 mg/kg and a 4 mg/kg dose, by the end of the second quarter. Initial results from this single-dose study fell short of investor expectations last October.
Last week the FDA notified Tekmira Pharmaceuticals that a partial clinical hold on the company’s Investigational New Drug application (IND) for TKM-Ebola had been modified to permit repeat dosing of healthy volunteers. The IND remains on partial clinical hold with regard to doses above 0.24 mg/kg/day. TKM-Ebola is a similar RNAi therapeutic, albeit for the treatment of Ebola. The drug is currently being studied in Sierra Leone.
One or more of PropThink’s contributors are long TKMR or ARWR.