Genzyme (of Sanofi [SNY]) announced on Wednesday a collaboration with the private gene therapy company Voyager Therapeutics to develop and commercialize novel therapeutics for severe neurological disorders.
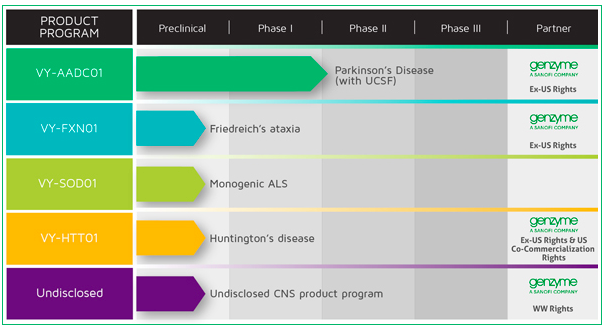
Voyager was launched just last year with a focus on adeno-associated virus (AAV) gene therapies for CNS disorders, including programs for Parkinson’s disease, Friedreich’s ataxia and Huntington’s disease, among others. The company was funded through a $45 million Series A round.
Gene therapy and the potential for one-time “cures” has captured the attention of Wall Street, most recently with the $161 million IPO of Spark Therapeutics (ONCE). We’ve written in more depth about fellow developers bluebird bio (BLUE) and uniQure (QURE) at PropThink, which you can read about here.
Genzyme will pay $100 million to Voyager up front, including $65 million in cash, a $30 million equity investment, and additional in-kind contributions. Voyager is eligible to receive development and sales milestone payments of up to $745 million, in addition to royalties on successful products. In aggregate, the deal could be worth $845 million.
Genzyme will have the option to license several programs following completion of an initial proof-of-concept human clinical trial. Voyager retains all U.S. rights to VY-AADC01, its lead program in Parkinson’s disease, and VY-FXN01 in Friedreich’s ataxia. Voyager will split U.S. profits with Genzyme on VY-HTT01 if successful in Huntington’s disease. VY-AADC01 is in an ongoing phase Ib study being run with U.C. San Francisco.
Voyager’s VY-SOD101 in amyotrophic lateral sclerosis is not part of the collaboration.