At a medical meeting this week Alnylam Pharmaceuticals (ALNY) reported six-month data from an ongoing study of patisiran (ALN-TTR02) in the rare disorder TTR-mediated amyloidosis (ATTR) in patients with familial amyloidotic polyneuropathy (FAP). At 6 months, the patisiran results are encouraging though not defining. Alnylam needs more patients and more time on the drug before calling the drug an orphan homerun.
The data are being presented at the American Neurological Association’s 2014 Annual Meeting.
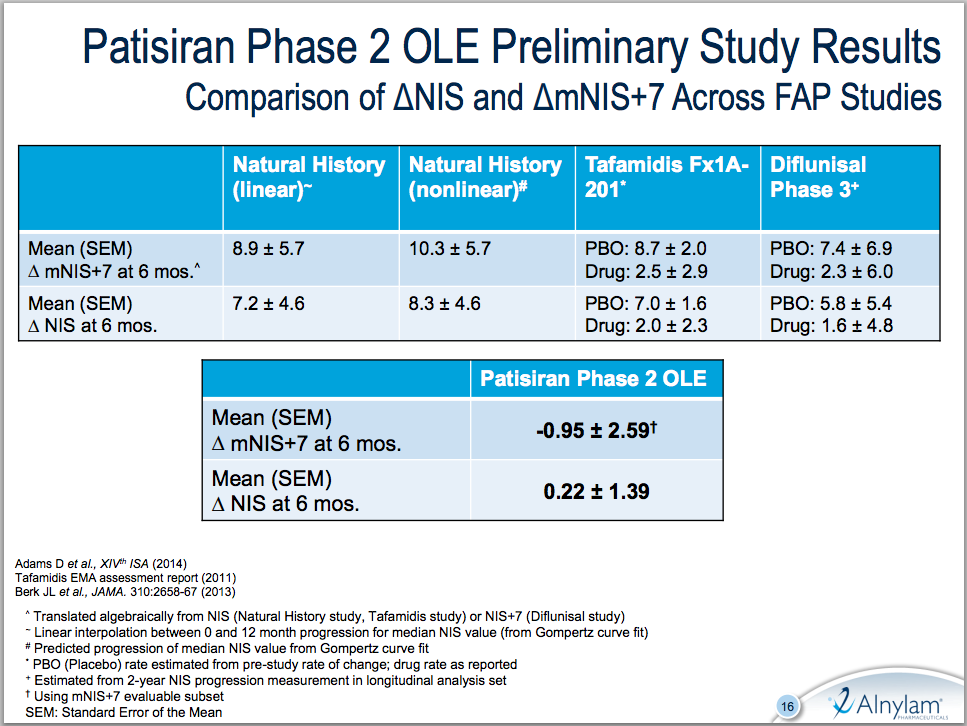
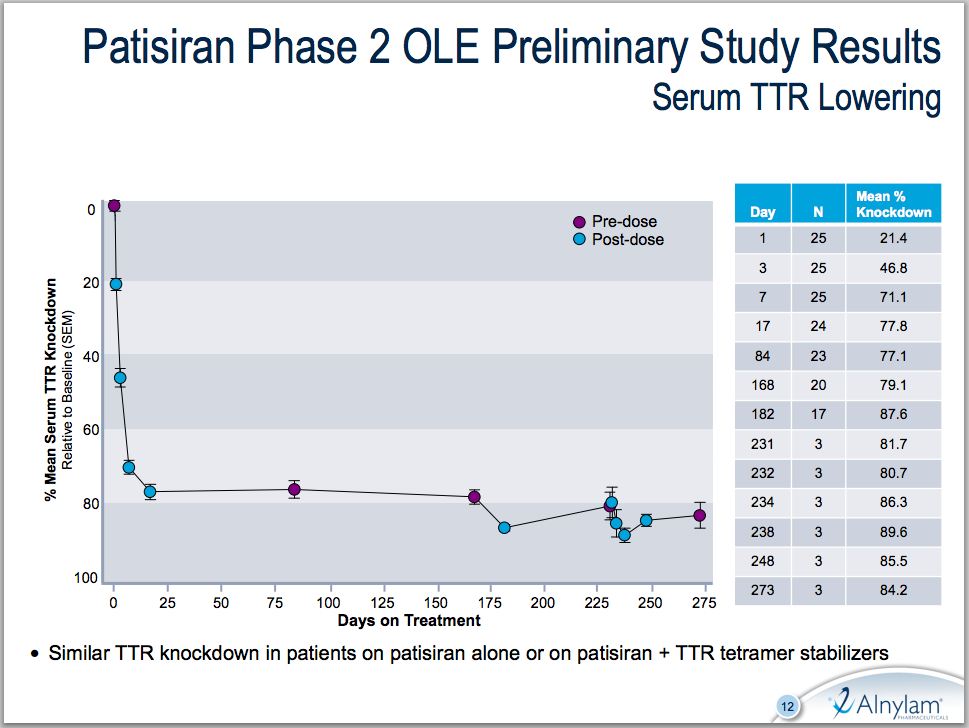
In the open-label FAP study, treatment with patisiran demonstrated on average a 0.95 point decrease in modified Neuropathy Impairment Score (mNIS+7) at six months in 19 patients. mNIS+7 is an evaluation of muscle weakness, sensory function, and nerve damage; neuropathy progression results in a higher score over time. Alnylam compares the 0.95 decrease to a 7 – 10 point increase in mNIS+7 at six months seen historically in untreated FAP patients. The study does not include a control arm, and though 27 patients have been enrolled, only 19 had completed 6 months of treatment and were evaluable for efficacy. Patisiran also produced a sustained mean TTR knockdown of 80% for over nine months, and up to 89.6% between doses. Alnylam reported no drug-related serious adverse events to date.
In the slide below, Alnylam compares mNIS+7 results with patisiran to a number of historical studies. Note the wide deviations on mNIS+7 results, particularly in the phase 3 diflunisal study.
Alnylam initiated a phse 3 study of patisiran, dubbed APOLLO, in November of last year. The 200-patient placebo-controlled trial uses mNIS+7 at 18-month as a primary endpoint.
The data this week, particularly patisiran’s safety, also bode well for the smaller RNA interference company, Tekmira Pharmaceuticals’ (TKMR), whose 2nd-gen LNP delivery technology enables patisiran. This in combination with the first domestically infected Ebola patient over the weekend should put momentum behind TKMR early this week.
One or more of PropThink’s contributors are long TKMR.