Agios Pharmaceuticals (AGIO) announced on Tuesday the initiation of a phase I/II multicenter study of AG-221 in patients with advanced solid tumors that carry an IDH2 mutation. This is the second trial to be initiated as a part of AG-221’s clinical development program, along with an ongoing phase I trial in hematologic malignancies.
AG-221 is a first-in-class inhibitor of mutant IDH2. The inhibition of IDH2 leads to a decrease in the production of 2-Hydroxyglutarate (2-HG), and is a metabolic driver of many tumors. This mechanism of action has been confirmed in the phase I trial of AG-221 in IDH2 mutant positive hematologic malignancies. Data presented from this phase I trial at the 19th Congress of the European Hematology Association (EHA) in June demonstrated an Objective Response rate of 56% (14/25) and a Complete Response rate of 24% (6/25), extremely compelling for a phase I trial in pretreated patients with difficult-to-treat cancers (AML/MDS), in which the key objective was not signs of clinical efficacy, but instead to determine -221’s safety and optimal dosing regimen.
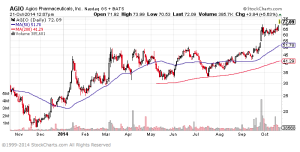
The stock’s reaction to Tuesday’s announcement regarding a phase I/II in solid tumors, and AGIO’s performance in the last few months, demonstrates investor appetite for news flow from this in-demand oncology company, and for companies with strong management teams developing drugs that can redefine the treatment paradigm in difficult oncology indications, much like Pharmacyclics (PCYC) has done with Ibrutinib. It would appear investors are positioning themselves to own AGIO ahead of key clinical data releases towards the end of the year.
AGIO is presenting clinical data from their other IDH mutant inhibitor, AG-120, at EORTC-NCI-AACR 2014 on November 18-21. In addition, AGIO will present additional data from the phase I trial of AG-221 in hematologic malignancies, and data from their AG-348 program, at the 56th Annual American Society of Hematology meeting December 6-9, 2014.
One or more of PropThink’s contributors are long PCYC.