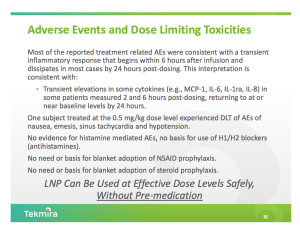
The US FDA has placed Tekmira Pharmaceuticals’ (TKMR) phase one study of TKM-Ebola, testing the drug candidate’s safety in healthy volunteers, on clinical hold following observed immune stimulation at higher doses. TKMR dropped 15% on the news, though there’s reason to believe Tekmira can alleviate this safety issue.
The FDA requested additional data related to cytokine elevations, which were observed at higher doses of TKM-Ebola in the current single ascending dose portion of the study, before allowing Tekmira to advance to the multiple ascending dose portion of the study. The company is also working on a change to the study protocol for patient safety, though the specifics are unclear.
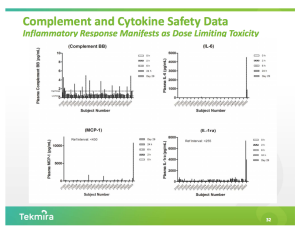
Cytokine elevations became apparent in May when Tekmira presented an update to the current trial at the Annual Meeting of the American Society of Gene and Cell Therapy.
Likely at issue is Tekmira’s choice to forego immune suppression or prophylactic pre-medication before TKM-Ebola dosing. In previous generations of Tekmira’s LNP delivery technology, the company pre-medicated patients (steroids, etc) to avoid problematic immune response at infusion (recall that the current TKM-Ebola program employs Tekmira’s 3rd-gen technology). Tekmira went out on a limb by skipping pre-medication in this study, which was largely thought to be incongruent with viral infection. Temporarily suppressing immune response before dosing could present a simple fix. If that’s the case, investors taking the long bet on Thursday’s weakness could be rewarded for some foresight.
RNAi consultant and blogger Dirk Haussecker in May penned a detailed analysis of the cytokine reaction at The RNAi Therapeutics Blog.