- Cash backstop and battered stock make Conatus (CNAT) an attractive long-term idea ahead of second half proof of concept for lead drug candidate, emricasan.
- Conatus’ enterprise value is less than 15% of price paid by Pfizer, Inc (PFE) to acquire emricasan nearly ten years ago. Clinical progress has been made since, and safety concerns that caused PFE to sell the asset now appear benign.
Conatus Pharmaceuticals (CNAT) has risen and fallen on parallel developments for Intercept Pharmaceuticals’ (ICPT) obeticholic acid (OCA). A series of favorable updates in the next 12 months, including phase 2 results of lead drug candidate, emricasan, in patients with nonalcoholic fatty liver disease (NAFLD) or fibrotic nonalcoholic steatohepatitis (NASH), will allow investors to better assess the commercial opportunity of the asset and may finally free the stock from Intercept’s shadow.
CNAT peaked at $15.67 on January 10, the same week that Intercept’s stock surged more than 280% after reporting that OCA, a first-in-class FXR agonist, had a significant beneficial effect on liver damage due to NASH (see: FLINT trial).
Lingering safety concerns and trial enrollment delays, however, have pressured CNAT; the stock recently closed below $5.50, down some 45% from its July 2013 IPO (six million shares priced at $11.00 each), and below pre-ICPT levels. With a number of value-driving events in the next year, Conatus presents an interesting way to be involved in NASH without owning the $6B Intercept. 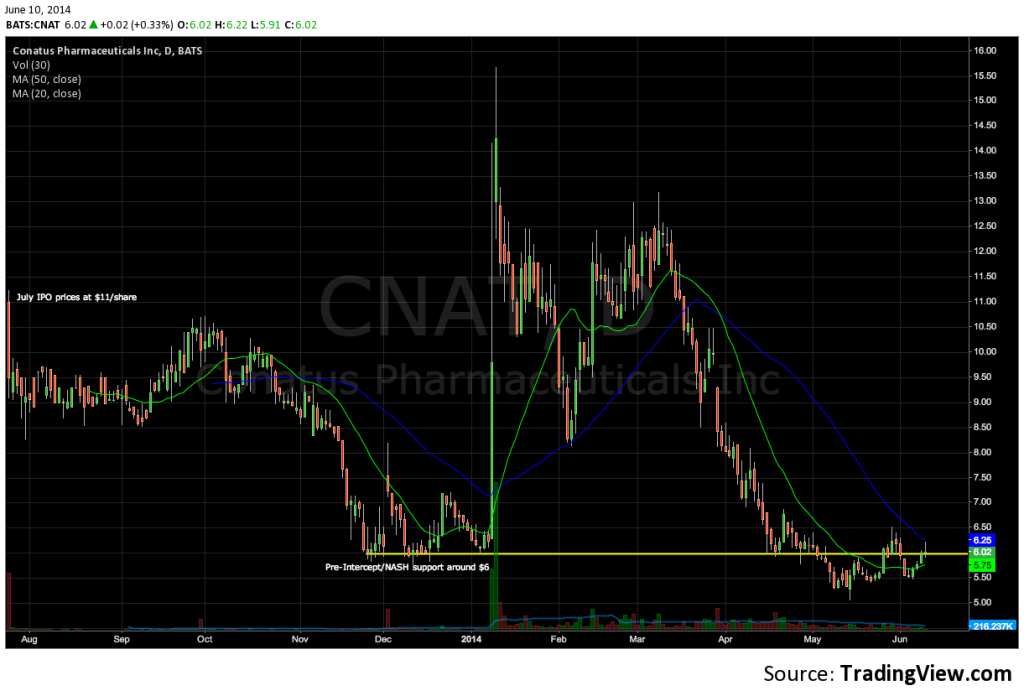 CNAT’s lead product, emricasan is a first-in-class, orally active pan-caspase protease inhibitor designed to reduce the activity of the caspase family of related enzymes that mediate inflammation (measured as serum ALT) and cell death, or apoptosis (measured as biomarker cCK18).
CNAT’s lead product, emricasan is a first-in-class, orally active pan-caspase protease inhibitor designed to reduce the activity of the caspase family of related enzymes that mediate inflammation (measured as serum ALT) and cell death, or apoptosis (measured as biomarker cCK18).
After initial success in a phase 2 trial of patients with chronic HCV infections and liver fibrosis, the pan-caspase inhibitor’s owner at the time, drug-maker Pfizer (PFE), halted work on emricasan in 2008 when U.S. regulators put a clinical hold on the drug due to observed inflammatory infiltrates during a study in mice the previous year.
Amid a massive R&D overhaul, Pfizer sold back the global rights to emricasan – originally purchased in July of 2005 for approximately $298 million – to a group that included the original sellers (CNAT’s co-founders) for an undisclosed sum in July 2010. Investors should note that CNAT’s current enterprise value is $39.07 million, roughly 13% of the priced valuation for the original emricasan clinical program a decade ago.
After re-acquiring emricasan, Conatus management met with the FDA for guidance on reinitializing clinical development. A 26-week carcinogenicity study in a murine model (predisposed to tumor development) that reproduced the previously observed findings of inflammatory infiltrates found no evidence of tumorigenicity. FDA’s sign-off on a new emricasan IND in January of 2013 effectively lifted the clinical hold on emricasan.
Though it’s been known for more than a decade that emricasan improves liver function (reductions in serum ALT and cCK18 levels) and appears to be an attractive therapeutic candidate against ischemia-reperfusion injury in liver transplants, the seemingly plodding pace from animal models to human testing has frustrated stockholders:
- In the third quarter of 2013, the initiation of a previously planned two-year Phase 2b/3 clinical trial in post-orthotopic liver transplant patients who have developed fibrotic injury due to Hepatitis C virus infection (HCV-POLT) was delayed, pending further analysis of the impact of Gilead Science’s (GILD) new HCV antiviral Solvadi (sofosbuvir) on CNAT’s ability to recruit and maintain patient compliance during the proposed two years of dosing. In the interim, however, the FDA did grant emricasan orphan drug status (with the requisite guerdons of R&D tax incentives, exemption of prescription drug user fees, and marketing exclusivity for 6 – 10 years) for the treatment of liver transplant recipients with reestablished fibrosis – to delay the progression to cirrhosis and end-stage liver disease – last December. Enrollment in the U.S. arm of the study is now underway.
- In March 2014, management admitted to delays in a previously planned phase 2b dose-ranging study [initial U.S. recruitment – January 2014] in acute-on-chronic liver failure (ACLF). The enrollment criteria, which initially focused on a precisely defined subpopulation of critically ill patients, proved to be too restrictive for timely patient recruitment and delayed completion of the 28-day trial (as pathophysiology remains complex and poorly understood, there is no accurate definition or physician consensus of ACLF as a clinical entity!). Ergo, the company has amended the protocol and revised inclusion and exclusion criteria to address the most common causes for patient screening failures.
Astute investors have judicious cause to look askew at CNAT, for now. Management promulgates that, “to date, emricasan has been studied in over 500 subjects in ten clinical trials without incident.“ Six of these studies were phase 1 studies comprised predominantly of healthy volunteers; the four phase 2 trials conducted here in the U.S. and in the E.U. principally focused on liver disease patients with HCV. That said, the studies have consistently demonstrated that emricasan results in reproducible reductions in elevated biomarkers of inflammation (such as ALT) and apoptosis (cCK18). Value-creating catalysts in the next 12-months:
- Interim data on the HCV-POLT study is expected in 1H15. Emricasan slowing fibrotic expansion (a sign of liver disease progression) based on the Ishak scale (which assesses liver fibrosis in 7 categories, ranging from normal to cirrhosis) would prove newsworthy.
- A 60-patient, 28-day PK study in ACLF patients should yield data in the second half of 2014 from a subset of patients with either severe renal impairment or hepatic impairment. Look for confirmatory dosing info and more importantly, time to clinical worsening (defined as the first occurrence of liver transplant, progression to next organ failure or death).
- Emricasan has already demonstrated an ability to reduce hepatic injury and liver fibrosis in murine models of cholestatic liver disease. Now, the drug must finally prove its therapeutic mettle in NAFLD and NASH, too. A 40-patient phase 2 NASH study is recruiting participants. End-of study histological biopsies aren’t an endpoint measure (as in Intercept’s FLINT study); however, confirmatory dosing and serum levels of injurious surrogate markers should be announced by fourth quarter 2014, readying the drug for phase 3.
Readers should be made aware that the use of surrogate endpoints in liver disease trials instead of “hard endpoints” is not necessarily a limiting factor: chronic liver diseases develop over many years or decades; consequently, surrogate markers must be relied on.
Intercept currently carries a market capitalization of $6 billion – CNAT, $93.8 million. Neither company is expected to turn a profit this year or next, and CNAT currently carries $3.00 per share in cash – Intercept, $6.36 a share (on a $288 stock!).
Why the disparity?
First-to-market and good press. Assuming Intercept’s OCA/cholesterol issue does not become a safety issue, OCA could enter a market with billions in sales potential by year-end 2015. According to recent epidemiological studies, NAFLD and NASH are the fastest growing liver diseases in the Western world, affecting approximately 12% of the U.S. adult population. Additionally, NASH is one of the leading causes of cirrhosis in adults in the United States, and up to 25% of adults with NASH may have cirrhosis. With no FDA-approved treatment for NAFLD or NASH currently available, one can see why Intercept carries a $6 billion valuation.
As discussed, CNAT shareholders need not depend on emricasan duplicating Intercept’s successful NASH program to move the dial higher: Conatus management estimates emricasan’s target indications in the United States and the EU at 150,000 ACLF patients, 10,000 CLF patients and 50,000 HCV-POLT patients.
At March 31, CNAT had net-cash equivalents of $50.1 million. Management expects to exit 2014 with a projected balance of $28 million to $32 million, which means the company should reach meaningful clinical milestones before tapping the markets for a secondary stock offering.
CNAT management emphasizes that emricasan is more than a bile-acid regulator (like Intercept’s OCA). It is a pan-caspase protease inhibitor that reduces all caspase activity, thereby reducing inflammation AND apoptosis activity, theoretically slowing the progression of liver disease. The science sounds good, but it will take real proof-of-concept to impress skeptical buyers – institutional ownership is markedly low, largely a result of PFE giving up on the asset last decade.
Also worth noting, CNAT has just 15M shares outstanding, less than 9 million in the float.




